Chemistry Reference
In-Depth Information
5.7.7 Electrochemical Fluorination Using
Inorganic Fluoride Salts
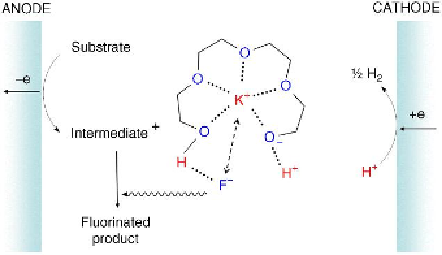
Inorganic fluoride salts such as alkali-metal
fluorides (MFs) are stable, easy to handle and
inexpensive. They are therefore strong
candidates for reagents in nucleophilic
fluorination as well as supporting electrolytes in
chemical and electrochemical fluorination. The
challenge is to overcome problems such as poor
solubility and low nucleophilicity of MFs in
organic solvents. Phase-transfer catalysts such
as crown ethers and quaternary ammonium or
phosphonium salts are known to reduce the
coulombic interactions of MFs and are
commonly used for this purpose. Fuchihgami
and co-workers reported successful anodic
fluorination in combination with an
electrochemical method using a poly(ethylene
glycol)/MF system where the MF is either KF or
CsF, as shown in
Figure 5.17
[88].
Search WWH ::

Custom Search