Chemistry Reference
In-Depth Information
acts as a kind of Lewis acid and is widely
applicable to various organic reactions, for
instance isomerization, transformation of
functional groups, carbon-carbon bond
formation, including Diels-Alder reaction, and
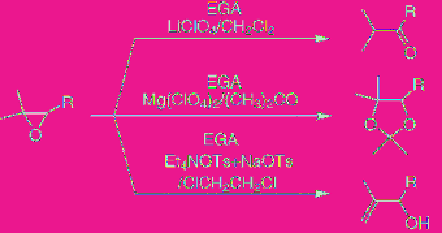
so on [31]. As shown in
Eq. 5.14
, a combination
of supporting salts and solvents allows three
kinds of products to be obtained selectively
from the same starting material [30].
(5.14)
Furthermore, EGAs are also highly effective for
the molecular transformation of organofluorine
compounds. For instance, as shown in
Eq. 5.15
,
the α-cation attached to the CF
3
group is
catalytically generated by the treatment of
trifluoromethylated
O,S
-acetal with an EGA,
and subsequently carbon nucleophiles are
readily introduced to the α-position [32].
However, use of other conventional Lewis acids
results in no formation of the desired product.
Search WWH ::

Custom Search