Geoscience Reference
In-Depth Information
as a result of decomposition of organic material is
also evident in sediment porewaters, which in the
top few millimetres to several decimetres of the
sediments typically have much higher
C
T
and lower
pH (Fig. 7.1), and, with increasing depth, generally
lower SO
4
2-
and higher sulphide concentrations
compared with the overlying bottom waters. These
trends are the direct result of microbial activity in
the sediments, but also the activity of large deposit
feeders that directly consume organic material and
indirectly affect microbial populations and geo-
chemical distributions of dissolved porewater
chemical species by mixing the sediments (see
Chapter 9 ).
Decomposition of organic material can be a prom-
inent process in the benthic boundary layer and at
shallow depth in sediments, since all particulate
organic material sedimented from the sunlit surface
and not decomposed during transit to the bottom is
either decomposed or buried here. In the coastal
ocean, this accumulation of organic matter may be
equivalent to most of the particulate organic mate-
rial produced in the surface water or deposited via
rivers and terrestrial run-off. In the open ocean, only
a few per cent of the particulate organic material
produced in the surface makes it to the benthos. For
the oceans as a whole, less than 1% of marine net
carbon production is preserved in sediment accu-
mulations (Mackenzie and Lerman 2006).
As a result of rising atmospheric CO
2
and the
enhanced greenhouse effect, air and seawater tem-
peratures are increasing. Warmer seawater temper-
atures may lead to increased metabolic rates,
resulting in higher rates of respiration and decom-
position of organic material, and consequently a
greater l ux of CO
2
from this process. Furthermore,
if increasing CO
2
is fertilizing net carbon produc-
tion in surface seawater (e.g. Riebesell
et al
. 2007 ),
the amount of organic material deposited in both
shallow and deep-sea sediments, and subsequent
pH
NBS
8.0
7.8
7.6
7.4
0
4
(A)
(B)
5
3
10
2
15
1
C
T
pH
2.2
2.6
3.0
3.4
1
2
3
4
C
T
(mmol kg
-1
)
(C)
(D)
20 mm
50 mm
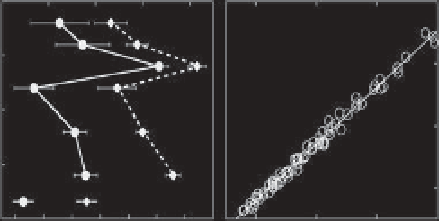
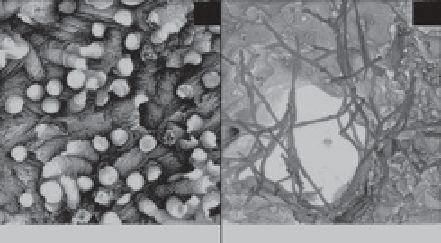
Figure 7.1
(A) Changes in daily average porewater pH
NBS
and total dissolved inorganic carbon (C
T
) as a function of sediment depth in shallow-water
(<1 m) carbonate sediments in Bermuda. Data were collected every 2 h at each depth for a complete diel cycle. Error bars represent one standard deviation.
(B) Changes in total alkalinity (A
T
) as a function of C
T
in shallow-water carbonate sediments in Mangrove Bay, Bermuda. The slope of a best i t line is close to
1, illustrating that the observed changes are due to metabolic dissolution of carbonate sediments. (C), (D) Visual evidence of microbioerosion in dead coral
substrate owing to microendoliths (from Tribollet 2008, with kind permission of Springer Science + Business Media).