Biomedical Engineering Reference
In-Depth Information
The conventional approach to perform water vapor sorption kinetics is based on
the measurement of the film sample mass until reaching constant value corresponding
to a saturation level in a hydrated environment. For each hydrated environment, the
mass of sample is measured allowing to obtain the mass gain, expressed in percent,
or, in other word, the water concentration, expressed in mass of water sorbed per
mass of polymer or in mmol of water sorbed per mass of polymer, inside sample. The
combination of mass gain at each humidity level leads to build the water vapor sorp-
tion isotherm. The water vapor sorption and the isotherm shape are depending on the
moisture resistance of polymer and its ability to interact with water.
By using Saturated Salt Solutions
After a drying time under vacuum, the polymer films were kept in saturated water va-
pors in separate containers at constant temperature during a period of time necessary
for equilibrium saturation of the polymer film (Iordanskii, 1998, 1999).
Appropriate saturated salt solutions were used to provide constant water activities
(a
w
), or relative humidities, ranging from 0.05 to 0.98 according to standard UNE-EN
ISO 483:1988. Ideal behavior is assumed, when activity is evaluated as the ratio of
the water vapor pressure (p
w
) to the saturated water vapor pressure (p
sat
w
)
at 25°C i.e.:
a
w
= p
w
/ p
sat
w
.
Technically, samples were periodically weighed and water sorption equilibrium
was considered to be reached when no mass change occurred. Then, the water con-
centration in the films was calculated from the sorption data in the stated conditions.
This technique is less widely used for the benefit of automated dynamic gravimetric
sorption system.
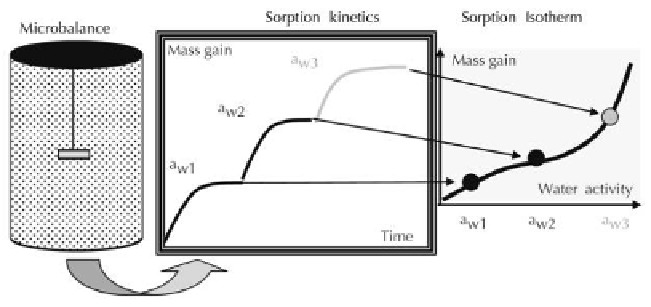
By using an Electronic Micro-balance
The water vapor sorption experiments were generally performed in a Cahn D-200
electronic microbalance (with a sensitivity of 10
-5
g) enclosed in a thermostated reactor
(Figure 5). The sample is placed in a pan and dried at 0% humidity. After reaching a
plateau, the dry mass is achieved. Thereafter, the sample is exposed to vapor pressure
and the mass gain is measured as a function of time until reaching the equilibrium
state. The mass equilibrium is obtained at each humidity level tested.
Figure 5.
Contribution of an electronic microbalance for building sorption isotherm (Follain, 2010).


Search WWH ::

Custom Search