Biomedical Engineering Reference
In-Depth Information
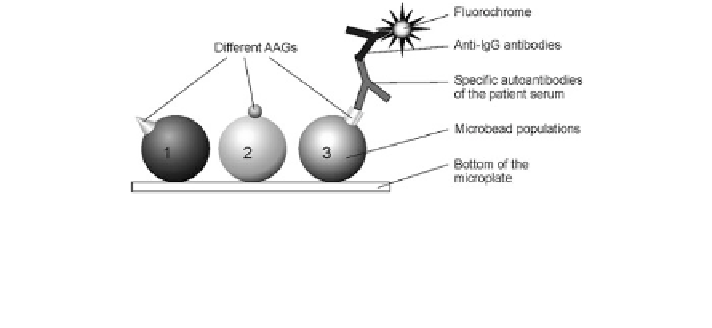
Fig. 11 Scheme of the antibody microbead assay. Three microbead populations (1, 2, 3) carrying
different autoantigens (AAGs) were immobilized on a microplate. Specific autoantibodies
(AABs) bind to their corresponding AAG (microbead 3) only. The reactive AAB are marked by
secondary fluorophore-labeled anti-IgG antibodies generating a ligand fluorescence on the
microbead surface
needs intensive quality assessment of each manufacturing step [
37
]. A suspension
microbead array [
25
] that uses microbead populations encoded by fluorescence
and/or size of their microbeads is a completely new approach. Each microbead
population carries a specific autoantigen (AAG) and by suspending different
microbead populations within one sample, a multiplex test can be performed.
However, a flow cytometer is required [
38
]. The aim of this study was to
characterize the analytic performance of the Attomol
ANA Microbead Assay
(ANA-BA) for qualitative multiplex detection of autoantibodies (AAB) using a
standard fluorescence microscope.
The antinuclear antibody microbead assay (ANA-BA) is provided as a ready-to-
use kit that provides all reagents for detection (Attomol GmbH; Bronkow; Germany).
It contains 11 different microbead populations, nine of which carry the AAGs
dsDNA, Sm, RNP/Sm, Ro60, Ro52, La/SS-B, Scl-70, CENP-B, and Jo-1 respec-
tively. In addition, a positive and a negative control population are included.
Microbead populations are mixed and permanently immobilized with PLL in a
standard 96-well microplate. Using four batches of the ANA-BA, two different
serum panels were analyzed to detect AABs according to the manual (Fig.
11
.) as
follows: (a) incubation of patients' serum diluted 1:50 in sample buffer, 1 h, (b) wash
buffer, three wash cycles each for 1 min, (c) fluorophore-labeled anti-human IgG
conjugate, 1 h, (d) wash buffer, four wash cycles each for 1 min, (e) automated
VideoScan measurement of relative fluorescence units.
All incubations were performed without agitation at ambient temperature.
Serum panel A comprises 100 sera of blood donors and panel B comprises 750
sera of patients with suspected autoimmune diseases. Since Jo1-AABs are extre-
mely rare, this parameter could not be used throughout the evaluation of test
sensitivity/specificity in the study.
Sera of panel B, exhibiting significant immune reactivity to one of the antigens,
were repeatedly analyzed to determine coefficient of variation (CV) [%] of intra-
and inter-assay, and inter-batch precision. To proof test stability of all microbead