Biomedical Engineering Reference
In-Depth Information
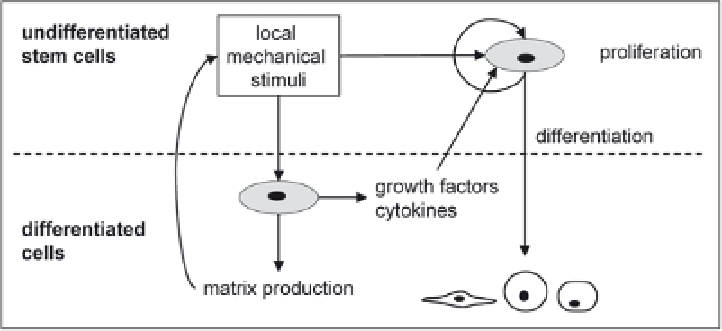
Figure 6. Tissue differentiation in fracture healing and DOG is affected by many factors. Here is depicted
a simplified scheme of how mechanical factors can play a role in this process. Mechanical load stimulates
cells to produce cytokines and growth factors, which affect cell differentiation and proliferation. It may also
directly stimulate the differentiation of a cell towards a certain phenotype. Mechanical load also stimulates
matrix production, which in turn affects the tissue properties and thereby the mechanical loads on the cells.
is influenced by mechanical loading. We are a long way from understanding the complex mul-
tifactorial process of fracture healing and DOG. Also the precise role of mechanical factors is
still poorly understood. On the one hand, the gradual stiffening and stabilization of the tissue
is required for ossification. On the other hand, tissue deformation plays a role in the stimula-
tion of cell proliferation, matrix production and the release of various growth factors. The
interaction of these factors result in the progressive development of the tissue (Fig. 6). Pres-
ently, in vitro experiments are only able to mimic aspects of this process. In vivo bone engineer-
ing is, however, a very promising practice. Several factors determining the success of bone
formation can be identified. These factors include the release of growth factors and cytokines,
the availability of a population of mesenchymal stem cells, good blood supply and appropriate
mechanical conditions. The future of bone engineering lies in the manipulation of these fac-
tors. The possibility of treatments using growth factors, such as BMP-2, has already been stud-
ied extensively.
125
In DOG, the appropriate cellular and chemical environment is created by
the callus tissue and the existing bone. Bone development is further stimulated by manipula-
tion of mechanical factors.
References
1. Wolff J. Das Gesetz der transformation der knochen. Translated as: The Law of Bone Remodel-
ling. Berlin, Germany: Springer Verlag, 1986.
2. Huiskes R, Ruimerman R, van Lenthe GH et al. Effects of mechanical forces on maintenance and
adaptation of form in trabecular bone. Nature 2000; 405(6787):704-706.
3. Frost HM. Bone Remodeling Dynamics. In: Thomas CC, ed. IL, USA: Springfield, 1963.
4. Weinans H, Sumner DR, Igloria R et al. Sensitivity of periprosthetic stress-shielding to load and
the bone density-modulus relationship in subject-specific finite element models. J Biomech 2000;
33(7):809-817.
5. Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop
1990; (250):8-26.
6. Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of
stability of fixation and soft-tissue preservation. Clin Orthop 1989; (238):249-281.
7. Jee WSS. Integrated bone tissue physiology: Anatomy and physiology. In: Cowin SC, ed. Bone
Mechanics Handbook. Boca Raton, FL, USA: CRC Press, 2001.
8. Parfitt AM. Osteonal and hemi-osteonal remodeling: The spatial and temporal framework for sig-
nal traffic in adult human bone. J Cell Biochem 1994; 55(3):273-286.
9. Palumbo C, Palazzini S, Zaffe D et al. Osteocyte differentiation in the tibia of newborn rabbit: An
ultrastructural study of the formation of cytoplasmic processes. Acta Anat (Basel) 1990;
137(4):350-358.
Search WWH ::

Custom Search