Biomedical Engineering Reference
In-Depth Information
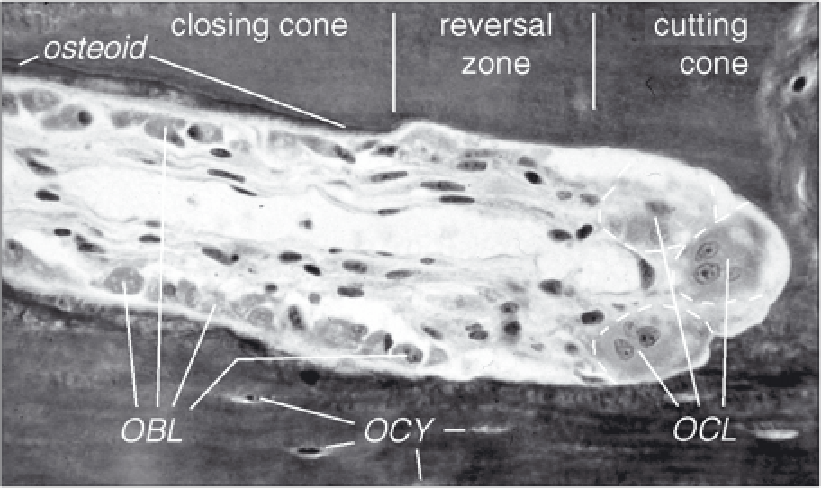
Figure 1. The cellular activity during bone remodeling. At the tip (cutting cone) multi-nucleated osteoclasts
(OCLs) excavate the mineralized bone tissue. At some distance, after the resting zone, osteoblasts (OBLs)
appear at the surface to refill the tunnel with osteoid that is subsequently mineralized. Osteocytes (OCYs)
are former osteoblasts that were entombed within the bone matrix, but remained connected to the bone
surface by numerous long slender protrusions (not visible). Typical outer diameter of an osteon in human
cortical bone is about 200
µ
m. Picture adapted from Schenk and Willenegger, 1964, courtesy R. Schenk.
connects the two bone surfaces is stretched, and as a result bone grows rapidly from the two
surfaces towards the distraction gap.
6
In this case the applied force causes strain not in the bone
itself, but in the soft osteogenic tissue that has filled the gap.
In the following chapter we will discuss currents concepts of the cell biology of these two
processes. We will argue that mechanotransduction in these cases is different, and that different
cell types are involved. We will then argue that the two most widely used models for applying
force on bone cells in culture, cell stretching and fluid shear stress, may represent each of these
two processes. In tissue engineering of bone, application of these force models during cell
growth in culture may each have their particular beneficial effect.
Mechanotransduction during Adaptive Bone Remodeling
In adult bone, osteoblastic and osteoclastic activity is largely confined to bone remodeling.
7
osteoclasts, large multinucleated cells related to macrophages, dig a tunnel in compact, cortical
bone or a trench along the surface of trabecular bone.
8
They are followed by osteoblasts, smaller
mononuclear cells that are related to fibroblasts and chondrocytes. The osteoblasts form
bone-specific extracellular matrix that calcifies, thereby replacing the old resorbed bone. Dur-
ing bone formation a number of osteoblasts differentiate into osteocytes and become entombed
in the new matrix.
9,10
The group of osteoclasts plus ensuing osteoblasts is called a Basic Multi-
cellular Unit or BMU.
3,11
The moving resorption front where the osteoclasts degrade existing
bone is called the cutting cone, and the tunnel or trench which the osteoblasts gradually fill
with new bone the closing cone (see Fig. 1). The new bone, organized as osteons in cortical
bone and hemi-osteons in trabecular bone, is aligned along the dominant local loading direc-
tion, suggesting local strain gradients as regulating principle.
12-14
This leads to the question
how strains are sensed in bone tissue, and by which cells. Many recent studies show that
mechanosensing in intact bone is primarily a task for the
osteocytes
, the mature, long-lived,
terminal differentiation stage of osteoblasts that lie buried in the mineralized bone matrix (see
Search WWH ::

Custom Search