Biology Reference
In-Depth Information
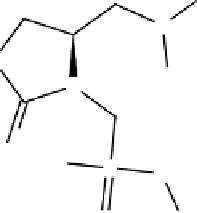
B) Anatoxin-a(s):
Anatoxin-a(s) is a unique N-hydroxyguanidine methylphosphatase ester with a
molecular weight of 252 D (Fig. 14; Matsunaga
et al
., 1989; Ressom
et al
., 1994). It has been isolated
from
A
.
fl os-aquae
and
A
.
lammermanni
. Mahmood and Carmichael (1986a) reported the production
of anatoxin-a(s) by
A
.
fl os-aquae
NRC 525-17. One of the important syndromes of anatoxin-a(s) is
salivation in vertebraes (Carmichael and Falconer, 1993). That is why the letter 's' is added as a
suffi x in parenthesis.
The biosynthesis of anatoxin-a(s) has been studied in
O
.
formosa
and all carbon atoms are
suggested to be derived from amino acids. L-Methionine is suggested to contribute the methyl
carbons or other donors to the C-1 pool whereas L-arginine accounts for C-2, C-4, C-5 and C-6
carbons of the toxin (Moore
et al
., 1992, 1993). Purifi ed anatoxin-a(s) has a LD
50
value of 50 µg kg
-1
body weight in a mouse bioassay. In acute toxicity tests of mice and rats, the signs of poisoning
included excessive cholinergic stimulation. In contrast to anatoxin-a, anatoxin-a(s) inhibits the activity
acetycholineterase. Complete inactivation of serum cholinesterase of rats was noted at doses of 350
and 600 µg kg
-1
body weight. Anatoxin-a(s) reacts with cholinesterase phosphorylating it and forms
a complex. As the enzyme is unable to degrade acetylcholine, overstimulation of muscle cells occurs
(Carmichael, 1997; Singh
et al
., 1999).
C) STXs
: These are the most widely known group of neurotoxins mainly produced by marine
dinofl agellate species of
Alexandrium
(Fig. 15 A),
Pyrodinium
and
Gymnodinium
(Fig. 15 B; Oshima
et al
., 1993) These toxins are chemically most diverse comprising of macrolides, cyclic polyethers,
sprolides and purine alkaloids. Their biosynthetic pathways and molecular genetics have been
summarized (Kellmann
et al
., 2010). The generic name 'saxitoxins' for the paralytic shellfi sh toxins
(PSTs) produced by freshwater cyanobacteria was proposed by Sivonen and Jones (1999). STXs are
tricyclic guanidium alkaloids (Fig. 16). There are more than 30 naturally occurring STX analogues.
STXs accumulated by shellfi sh when consumed by human beings results in PSP. Cyanobacteria
that are known producers of STXs are
A. circinalis
(Fig. 4C; Humpage
et al
., 1994),
Planktothrix
sp.
FP1 (Pomati
et al
., 2000) from Australia,
Aph
.
fl os-aquae
from USA (Alam
et al
., 1973; Mahmood and
Carmichael, 1986b) and Portugal (Pereira
et al
., 2000),
Aph
.
gracile
(Fig. 2 C) from northeast Germany
(Ballot
et al
., 2010) and France (Ledereux
et al
., 2010),
Aphanizomenon
sp. DC-1 from China (Liu
et al
.,
2006),
C. raciborskii
(Sawyer
et al
., 1968; Lagos
et al
., 1999; Bouvy
et al
., 1999; Bernard
et al
., 2003) and
R. brookii
D9 (Stucken
et al
., 2010) from Brazil and
L. wollei
(Carmichael
et al
., 1997) from USA are
known producers of PSTs. There appears to be a geographical segregation of strains of
A
.
circinalis
since Australian isolates are known to produce PSTs whereas in other continents the production
of other neurotoxins such as anatoxin-a and anatoxin-a(s) has been reported (Sivonen and
Jones, 1999).
CH
3
N
H
CH
3
N
+
O
-
H
2
N
P
O
O
CH
3
Figure 14:
Structure of anatoxin-a(s).