Biomedical Engineering Reference
In-Depth Information
CENTRAL NERVOUS SYSTEM
Cerebrospinal Fluid
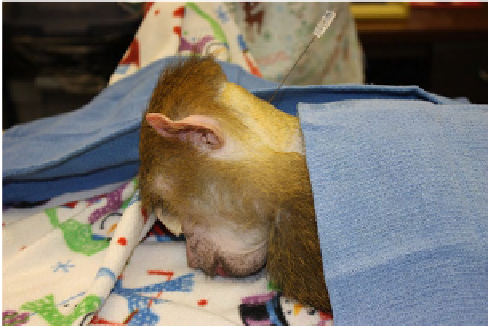
Cerebrospinal fluid (CSF) is typically collected from either
the cisterna magna or the lumbar subarachnoid space. A
detailed description of CSF collection from the common
marmoset via suboccipital puncture has been described
(
Geretschlager et al., 1987
). After anesthetization of the
marmoset and aseptic preparation of the skin surface, the
head is held in complete flexion. A 0.5-mm-diameter,
14-mm-long cannula from a disposable scalp vein set is
inserted 8 mm distal to the external occipital protuberance
and advanced until it touches the occipital bone. The cannula
is then redirected toward the posterior atlantooccipital
membrane, which is pierced to enter the cisterna magna.
CSF flows readily into a 1.0-ml syringe. This procedure may
be repeated multiple times without complication. A similar
approach using a 22-gauge, 1.5-inch spinal needle was
performed in cynomolgus monkeys (
Lipman et al., 1988
)
(
Figure 13.11
). In a report which compared CSF character-
istics from suboccipital and lumbar puncture sites in rhesus
monkeys, the animals were placed in ventral recumbency,
and a sterile 22-gauge hypodermic needle was inserted just
rostral to the arch of the atlas on the dorsal midline (
Smith
and Lackner, 1993
). Lumbar puncture is best performed with
the nonhuman primate in lateral recumbency but has also
been described in rhesus monkeys positioned in a restraint
chair (
Snead and LaCroix, 1977
). After positioning the
nonhuman primate, the area of the lower back is shaved and
prepped. The lumbar interspace at the same level as the iliac
crest is palpated and entered with a spinal needle. The spinal
needle is angled very slightly cephalad as it is advanced.
Sometimes a characteristic “pop” can be felt when the
needle penetrates the dura. The stylet is then removed and
the needle observed for fluid return. If no fluid is appreci-
ated, the stylet is reinserted and the needle advanced or
withdrawn until fluid flows.
MUSCULOSKELETAL SYSTEM
Intramuscular Injection
Intramuscular injections are one of the most common
routes of administering drugs to nonhuman primates. The
use of a squeeze cage makes this technique the method of
choice for delivering drugs that are available in intramus-
cular injection form. Small nonhuman primates may be
restrained by physical means. Typically either muscles of
the caudal thigh, cranial thigh, deltoid, or the longissimus
of the back are used. One must be familiar with the normal
anatomy of the primate and avoid injecting the intramus-
cular injection into an area where a blood vessel is located,
where a bone is located near the skin surface, or near
a nerve, e.g. the sciatic nerve must be avoided when
injecting into the caudal thigh muscles especially in small
nonhuman primates (
Brady, 2000
). One author (
Line,
1993
), quoting the human literature, suggests limiting the
volume for intramuscular injection at one site to 0.5 ml in
a nonhuman primate up to approximately 3 kg or 1.0 ml in
a nonhuman primate up to approximately 13 kg as a means
of lowering the risk of sterile abscess formation, muscle
contractures, and vascular compression injuries. The tip of
the needle is placed deeply into the muscle. The syringe is
aspirated to ensure that the needle is not in a blood vessel. If
blood is seen, the needle is withdrawn and discarded. When
placement is correct, the liquid is injected slowly to allow
the muscle fibers to stretch.
Skeletal Muscle Biopsy
Skeletal muscle biopsies can be obtained from numerous
muscles from the thigh, arm, or the back. The vastus muscle
group is often used when using the leg and both the biceps
and the triceps muscles of the arm work well for obtaining
muscle biopsies.
Bone Biopsy
Bone biopsies may be necessary in nonhuman primates.
For most clinical applications, percutaneous bone biopsy is
preferred. The biopsy specimen can be obtained using
a Jamshidi needle. The needle with stylet is advanced
through a small skin incision to the area of interest. At that
point the stylet is removed and then the needle is advanced
using a twisting clockwise and counterclockwise motion.
Once a core of sufficient length has been cut, it must be
broken off by rotating the needle several times in one
direction and then rocking it back and forth rather vigor-
ously. The needle is then removed and the biopsy tissue
pushed from the needle with the stylet. Goodwin and Jer-
ome reported on the technique for obtaining bone biopsies
in both baboons and cynomolgus monkeys (
Goodwin and
FIGURE 13.11
CSF collection from the cisterna magna. Some
drapes have been removed for better visualization.