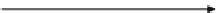Biomedical Engineering Reference
In-Depth Information
Internal Standard Infusion
HPLC
Column
Mobile Phase
MS
Mixer
Fig. 3
Schematic representation of continuous infusion of an internal standard and postcolumn
(postelution) mixing with LC eluent prior to being directed to a mass spectrometer (MS) for
detection
separation of free and liposome-encapsulated drug forms. Good interday precision
and accuracy were obtained, for example, % CV ranging from 4.20 to 8.26 (
n
= 84)
for free drug form and from 3.99 to 10.30 (
n
= 54) for liposome-encapsulated drug
form, which demonstrate the adequacy of this approach [
20
] . Similar approach was
also adopted by others for this type of compound [
21
] .
Apart from the above approaches, an internal standard can also be introduced
after chromatographic separation with the aim to mainly compensate for quantita-
tive errors attributed to signal suppression or enhancement in MS detection (Fig.
3
),
particularly when the sample loss during sample preparation procedure is minimal
or has been evaluated. This approach is useful to avoid the usage of multiple internal
standards in a multicomponent analytical method and to obtain the benefit of matrix
effect correction with coelution of an analyte and the internal standard (even with a
structural analogue internal standard). In addition, the quantity of the internal stan-
dard introduced may be readily controlled by adjusting the infusion flow rate and/or
the concentration of IS solution. It has been demonstrated by Choi et al. [
22
] that
one single SIL internal standard was good for both the parent drug and its metabo-
lite. However, despite the coelution of an analyte and its internal standard, how well
the matrix effect could be corrected will still depends on how close their physical-
chemical properties are, especially ionization related properties. In addition, an
additional pump is necessary for the introduction of the internal standard in this
case.
2.6
Alternative Approaches without an Internal Standard
When an appropriate internal standard could not be found, it is sometimes possible
to develop a method without an internal standard [
19
], especially for usage in early
drug discovery stage where less strict criteria could be used. Nevertheless, efforts
should be made to minimize the variations in sample extraction, LC separation, and