Biomedical Engineering Reference
In-Depth Information
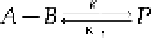
8.1.3 Single-Stage Reversible Chemical Reaction
Next, consider the single-stage reversible chemical reaction as given in Eq. (8.13):
ð
8
:
13
Þ
Here, the chemicals
A
and
B
react to form the product
P
, and
P
has a reverse reaction to
form
A
and
B.
The law of mass action describing this system is given by
q
P
¼
K
1
q
A
q
B
K
1
q
P
q
A
¼
K
1
q
A
q
B
þ
K
1
q
P
q
B
¼
K
1
q
A
q
B
þ
K
1
q
P
ð
8
:
14
Þ
Equation (8.14) is nonlinear, which can be solved using SIMULINK or also mathematically,
as shown in the following example.
EXAMPLE PROBLEM 8.1
Consider the reaction given in Eq. (8.14), with
q
A
ð
0
Þ¼
10,
q
B
ð
0
Þ¼
15, and
q
P
ð
0
Þ¼
0,
K
1
¼
2,
and
K
1
¼
3
:
Solve for
q
P
:
Solution
From Eq. (8.14), it is clear that
q
P
¼
q
A
¼
q
B
and after integrating, we have
q
P
q
P
ð
0
Þ¼
q
A
ð
0
Þ
q
A
¼
q
B
ð
0
Þ
q
B
ð
8
:
15
Þ
To solve for
q
P
, we eliminate
q
a
and
q
B
using Eq. (8.15) by substituting
q
A
¼
q
A
ð
0
Þþ
q
P
ð
0
Þ
q
P
and
q
B
¼
q
B
ð
0
Þþ
q
P
ð
0
Þ
q
P
into Eq. (8.14),giving
q
P
¼
K
1
q
A
q
B
K
1
q
P
¼
K
1
ð
q
P
ð
0
Þþ
q
A
ð
0
Þ
q
P
Þ
q
P
ð
ð
0
Þþ
q
B
ð
0
Þ
q
P
Þ
K
1
q
P
ð
8
:
16
Þ
Since
q
P
ð
0
Þ¼
0, we have
q
P
¼
K
1
ð
q
A
ð
0
Þ
q
P
Þ
q
B
ð
ð
0
Þ
q
P
Þ
K
1
q
P
¼
210
ð
q
P
Þ
ð
15
q
P
Þ
3
q
P
0
@
1
A
ð
8
:
17
Þ
53
2
q
P
þ
2
P
¼
2
q
150
and after rearranging terms
dq
P
dq
P
150
¼
Þ
¼
2
dt
ð
8
:
18
Þ
53
2
q
P
þ
ð
q
P
18
:
3
Þ
q
P
ð
8
:
2
q
2
P
Once again, partial fraction expansion is used to rewrite Eq. (8.18) as
dq
P
q
P
dq
P
q
P
0
:
0989
Þ
¼
2
dt
ð
8
:
19
Þ
ð
18
:
3
ð
8
:
2
Þ
Continued