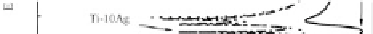Biomedical Engineering Reference
In-Depth Information
for Ti-Ag alloy with comparison to the CP Ti [57]. In the solution
containing 0.2% NaF (Fig. 5.12b), the corrosion potential of both CP
Ti and Ti-Ag decreases and the passivation current and corrosion
current densities increases, with respect to the luoride-free
solution. These imply a reduced corrosion resistance. In comparison
with CP Ti, the Ti-Ag alloys exhibit better corrosion resistance in
luoride containing solution too [57]. The pure Ti exhibit active-
passive transition pass in the luoride-containing solution. The
presence of F
−
in the solution retards the formation of passive ilm
on the surface of the Ti. The summarized corrosion parameters of
the CP Ti and Ti-Ag alloys, indicating higher corrosion resistance
of the Ti-Ag in artiicial saliva are presented in Table 5.2 [57]. In
the luoride solution, Na
2
TiF
6
or Ti F
6
2−
is formed, both of which
are more stable than TiO
2
, but unfortunately, they do not exhibit
protective, and the corrosion resistance of the Ti alloys decrease in a
luoride-containing solution [2, 57].
Figure 5.12
Potentiodynamic polarization curves of commercially pure Ti
and Ti-Ag alloys in artiicial saliva solutions [57].