Biomedical Engineering Reference
In-Depth Information
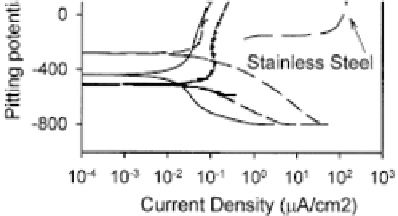
of 0.30 for bare Ni-Ti [47]. Rondelli and Vicentini [38] investigated
the Ni-Ti dental wires in artiicial saliva (Fig. 5.11). The Ni-Ti alloy
has a wide passive range that extends up to very high potentials, and
absence of hysteresis loop, which indicate high resistance to pitting
corrosion. For comparison, for the cobalt alloy no pitting occurs at
very high potential, whereas the stainless steel exhibited pitting of
about 400 mV versus SCE (Fig. 5.11). The Ti-Ni alloys have suficient
resistance against pitting corrosion, comparable to the cobalt-based
alloy, and much higher than the stainless steel wire [38].
Figure 5.11
Potentiodynamic tests of orthodontic wires in artiicial saliva
at 40
°C
[38].
Preparation of Ti-alloys with highly cathodic elements, such as
Ag, Pt, and Pd could improve corrosion resistance of the implant
alloys. Shim
et al
. [48] and Zhang
et al
. [57] reported that Ti-Ag
alloys have better corrosion resistance than that of pure titanium
and they predicted that Ti-Ag alloys are less sensitive to luoride
ions, which is extremely important in dental implant applications.
Zhang
et al
. [57] investigated the corrosion behavior of the Ti-Ag
alloys in artiicial saliva with luoride ions. Figure 5.12 shows the
potentiodynamic polarization curves of the commercially pure
Ti (CP Ti) and the Ti-Ag alloys in the artiicial saliva solutions. In
the luoride-free solution (Fig. 5.12a), both CP Ti and the Ti-Ag
alloys show passivation behavior without obvious active-passive
transition. The passivation and corrosion current density are lower