Biomedical Engineering Reference
In-Depth Information
Figure 17.
Contact angle of aqueous DTAB solution at the cmc on different polymer surfaces.
!
Teflon AF;
1
Parafilm;
E
PP;
PVF;
P
PET.
Figure 18.
Wetting activity of surfactants under study for polymer surface used.
surface. Using the wetting activity parameter, a suitable surfactant can be selected
for a given surface. The larger the value of the wetting activity, the more active is
surfactant. In our case, the most suitable surfactant for very hydrophobic Teflon AF
might be C
12
E
5
as shown in Fig. 18. For other surface under investigation, DTAB
may be used as the 'best' surfactant with respect to the wetting activity, apart from
other decision criteria.
Another parameter, the spreading rate, is of great significance in order to estimate
the spreading behaviour of a given surfactant on a given surface (cf. Section C.2).
Our results indicate that spreading, if it occurs, may be divided into two regimes:
I—the short time regime (fast spreading). In this regime, until approximately 1 s,
the base radius depends linearly on time; II—the long time regime (slow spreading).
In the long time regime, from 1 s to the equilibrium state, the radius values may
be fitted by a power function. Exponent value of this power function serves as a
measure of the spreading extent with respect to time. The larger the exponent value,
the faster the spreading as shown in Fig. 19.
The analysis of time dependencies of the drop base radius reveals that the slow
wetting dynamics observed for both ionic and non-ionic surfactants on hydropho-
bic surfaces can be explained neither by surfactant diffusion from the bulk of the
drop to the expanding liquid-vapour interface nor in terms of viscous spreading.
Two different explanations were provided [52]: First, a slow rearrangement of sur-













































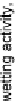




































































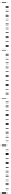




Search WWH ::

Custom Search