Biomedical Engineering Reference
In-Depth Information
Figure 16.
Effectiveness of surfactant solutions: a difference between the water contact angle and con-
tact angle of corresponding surfactant solution at the cmc; PVF: polyvinyl fluoride, PET: polyethylene
terephthalate.
(ii) the more hydrophilic the surface, the less effective is the respective surfactant;
(iii) with regard to the changes in wettability, the largest effects were obtained with
the non-ionic surfactant, especially on highly hydrophobic polymer surfaces.
From the experiment as shown in Fig. 17, polymer surfaces may be divided into
classes with reference to the spreading behaviour of aqueous surfactant solutions:
- highly hydrophobic surfaces such as Teflon AF, Parafilm and PP with the sur-
face free energy of 11.8, 18.4 and 23.2 mJ/m
2
, respectively;
- moderately hydrophobic surface such as PVF and PET with the surface free
energy of 36.2 and 36.7 mJ/m
2
, respectively.
For the highly hydrophobic surfaces, the contact angles for SDS, DTAB and
DTAS solutions do not change with time at any concentrations investigated. In con-
trast to the highly hydrophobic surfaces, the contact angles of the ionic surfactant
solutions on the moderately hydrophobic surfaces strongly depend on time. Simi-
larly to the behaviour observed for ionic surfactants on the moderately hydrophobic
surfaces, non-ionic C
12
E
5
spread well over both moderately and highly hydropho-
bic surfaces, but in the last case, the effect is less pronounced.
For the highly hydrophobic surfaces, the contact angles for SDS, DTAB and
DTAS solutions do not change with time at any concentrations investigated. In con-
trast to the highly hydrophobic surfaces, the contact angles of the ionic surfactant
solutions on the moderately hydrophobic surfaces strongly depend on time. Simi-
larly to the behaviour observed for ionic surfactants on the moderately hydrophobic
surfaces, non-ionic C
12
E
5
spread well over both moderately and highly hydropho-
bic surfaces, but in the last case, the effect is less pronounced.
An important parameter to compare the wetting behaviour of surfactant solutions
is the wetting activity (or wetting power), calculated from the derivative of the equi-
librium contact angle with respect to relative concentration (c/cmc). This quantity
relies not only on the surface activity of a surfactant at the liquid-vapour interface,
but it also depends on molecular interactions between the surfactant and a solid





























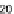

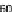







































































Search WWH ::

Custom Search