Biomedical Engineering Reference
In-Depth Information
solubility of such hydrophobically modi
ed alginates is strongly reduced as ionic strength
increases. Particles can then be formed by dropping a solution containing the polysaccharide
into a Na
+
solution or into a solution containing Na
+
and Ca
2+
. Appropriate conditions to
induce gel particle formation, and avoid polymer precipitation, were determined by adjusting
concentrations of NaCl, CaCl
2
and amphiphilic alginates. Amphiphilic derivatives of Na
+
alginate were prepared by esterifying long alkyl chains (12 or 18 carbon atoms) on to the
hydrophilic backbone. Highly viscous solutions and strong gels were obtained in water,
within a narrow range of substitution degree, i.e. 3
-
5% and 13
-
16% for C
18
-andC
12
-
modi
ed alginates, respectively, since at higher degree of substitution the polymers were no
longer water-soluble. The microstructure of Ca
2+
modi
ed alginate gels was more hetero-
geneous than that of Ca
2+
unmodi
ed alginate gels, because the dense hydrophobic domains
restrict egg-box formation (
Chapter 5
).
Another method of preparing microparticles from hydrophobically modi
ed alginate
derivatives uses an
procedure (Leonard et al.,
2004
); see
Figure 11.11
. The
main problem arising from the use of amphiphilic alginates is the high pre-gel viscosity
of the solution, so dispersion in NaCl solution was dif
'
all-aqueous
'
cult and particle sizes much higher
than required.
Figure 11.11
Schematic representation of the process used for preparation of microparticles of amphiphilic
alginate. Adapted from Leonard et al.(
2004
) with permission from John Wiley & Sons.














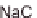

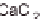
















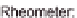














































Search WWH ::

Custom Search