Biomedical Engineering Reference
In-Depth Information
(
c
)
(
a
)
(
b
)
1
μ
m
1
μ
m
1
μ
m
(
f
)
(
d
)
(
e
)
1
m
1
m
1
m
μ
μ
μ
Figure 13.5
Figure 13.13 (Con.)
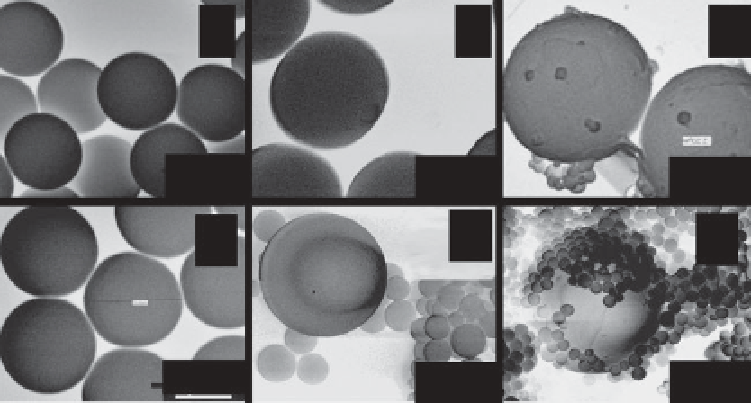
The SEM image of MMS synthesized in which the effect
on the morphologies in ternary surfactant system; (a-e) are the SEM images of
samples 2-A, 2-B, 2-C, 2-D, 2-E respectively. The scale bar, a-e, 1 μm; (f) is the
lower magnifi cation SEM image of sample 2-C, the black: 5 μm. (Figure 13.5)
a~f, The SEM image of MMS synthesized in binary surfactant system, in which
the effect on the morphologies via changing the proportion of co-solvent is
displayed; the scale bar: a-f, 1 μm Right: the plots of the size of the MMS to the
co-solvent ratio. The scale bar: a-f, 1 μm. (Reprinted with permission from [73])
by adding alkyltrimethyl-ammonium bromide as templates to control
porosity, and the spheres had large specifi c surface area and an average
diameter of nearly 700 nm [71]. Comparatively, Stöber's method in alka-
line medium is still a prior choice in the synthesis of the monodispersed
and uniform porous spheres, but there are few reports about the study
on the synthesis of the porous spheres with diameter above 1.0 μm using
this approach. Yano and Fukushima have synthesized the monodispersed
mesoporous silica (MMS) sphere with a size of about one micrometer by
adding C
n
TMACl (C
18
TMACl, C
16
TMACl, and C
14
TMACl) as surfactants
and TMOS as silica source in the alkaline medium with methanol and
water as co-solvent, and this is already a breakthrough [72].
We will now introduce a one-step preparation of the MMS of silica with
a uniform diameter above 1.0 μm by introducing a mixture of cationic sur-
factant and nonionic surfactant to sol precipitation [73]. The effects on the
morphologies of the MMS were investigated by changing the proportion
of the surfactant as well as that of the solvent, and the optimal condition
for synthesis of the lager MMS in this system was found. A notable feature
of the sphere is its large diameter and uniformity. The spheres obtained in
CTAB\ dodecylamine (DDA) system have a diameter of nearly 1.20 μm,
Search WWH ::

Custom Search