Biomedical Engineering Reference
In-Depth Information
each disaccharide (
Fig. 7.2-4
). It is a constituent of ECM,
contributing to the functionality of the extracellular
network. The cartilage ECM consists of type II collagen
and proteoglycans including aggrecan, which are re-
sponsible for the tissue's compressive and tensile
strength, respectively. Chondroitin sulfate forms the
arms of the aggrecan molecule in cartilage.
factors to the cells. The ECM is a complex structural
protein-based entity surrounding cells within mammalian
tissues. Most normal vertebrate cells cannot survive
unless they are anchored to the ECM. In tissues and
organs, major ECM components are structural and
functional proteins, glycoproteins, and proteoglycans
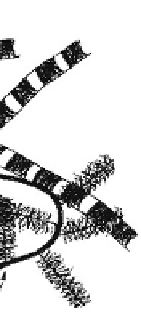
arranged in a unique, specific 3-D ultrastructure, as il-
lustrated in
Fig. 7.2-6
. Each tissue or organ has its own
unique set and content of these biomolecules. In skin, the
collagen:elastin ratio is about 9:1, whereas in an artery
this ratio is 1:1 averaging all artery layers, and 1:9 when
considering the lamina elastica only. In ligaments, the
collagen:elastin ratio is also 1:9, and in lung about 1:1.
Likewise, the amount and type of GAGs, another major
ECM component, varies from matrix to matrix. For in-
stance, in cartilage CS is the major GAG making up 20%
of the dry weight. In skin, dermatan sulfate is the most
abundant (about 1% of the dry weight), whereas in the
vitreous body of the eye the major GAG is hyaluronate.
Natural ECMs are gels composed of various protein
fibrils and fibers interwoven within a hydrated network
of GAG chains. In their most elemental function, ECMs
thus provide a structural scaffold that, in combination
with interstitial fluid, can resist tensile (via the fibrils)
and compressive (via the hydrated network) stresses. In
this context it is worth mentioning just how small a pro-
portion of solid material is needed to build mechanically
quite robust structures. Structural ECM proteins include
collagensdsome of which are long and stiff and thus
serve structural functions whereas others serve connect-
ing and recognition functionsdand elastin, which forms
an extensive crosslinked network of elastic fibers and
sheets. The anisotropic fibrillar architecture of natural
ECMs has apparent consequences for cell behavior. Be-
cause of a tight connection between the cytoskeleton and
the ECM through cell surface receptors, cells sense and
respond to the mechanical properties of their environ-
ment by converting mechanical signals into chemical
Chitosan and chitin
Chitosan is a linear polysaccharide of (1-4)-linked
D
-glucosamine and
N
-acetyl-
D
-glucosamine residues de-
rived from chitin, which is found in arthropod exo-
skeletons (
Fig. 7.2-4
). The degree of N-deacetylation of
chitin usually varies from 50% to 90% and determines
the crystallinity, which is the greatest for 0% and 100%
N-deacetylation. Chitosan is soluble in dilute acids which
protonate the free amino groups. Once dissolved,
chitosan can be gelled by increasing the pH or extruding
the solution into a non-solvent. Chitosan derivatives and
blends have also been gelled via GA crosslinking, UV ir-
radiation, and thermal variation. Chitosan is degraded by
lysozyme, and the kinetics of degradation is inversely
related to the degree of crystallinity.
Figure 7.2-5
shows
the dependence of resorption of chitin on the hydrolysis
extent when partially hydrolyzed chitin (or partially
acetylated chitosan) is subcutaneously implanted in rat
[2]
. In contrast with 100% homopolymeric chitosan,
partially hydrolyzed chitin or partially acetylated
chitosan and chitin are absorbable and high in the tensile
strength, but it seems that clear evidence has not yet
been presented regarding its safety, especially when
implanted in the human body.
7.2.2.1.3 Natural composite—ECM
The native ECM provides a substrate containing adhesion
proteins for cell adhesion and regulates cellular growth
and function by presenting different kinds of growth
30
20
10
Chondroitin sulfate
0
60
Degree of deacetylation (mol%)
80
100
Collagen fibril
Fig. 7.2-5 Dependence of the initial resorption rate on films
of chitin and its deacetylated derivatives on the degree of
deacetylation.
Hyaluronic acid
Fig. 7.2-6 Component arrangement in ECM (cartilage).