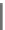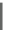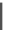Biomedical Engineering Reference
In-Depth Information
COOH
CH
2
OH
COOH
O
O
O
O
O
COOH
HO
O
OH
O
OH
HO
O
OH
HO
NHCOCH
3
n
m
OH
-D-mannuronic acid
-L-guluronic acid
β
α
Hyaluronic acid (HAc)
Alginate
COOH
CH
2
OSO
3
H
CH
2
OH
O
HO
O
O
O
O
OH
O
OH
OH
NHCOCH
3
NHCOCH
3
Chondroitin sulfate (CS)
Chitin
CH
2
OH
CH
2
OH
O
O
O
OH
OH
O
NH
2
OH
Chitosan
Starch
Fig. 7.2-4 Chemical structure of polysaccharides used for tissue engineering.
Oligosaccharide HAc (o-HAc) fragments have been
shown to induce angiogenesis in several animal models as
well as within
in vitro
collagen gels. HAc is naturally
hydrolyzed by hyaluronidase, allowing cells in the body
to regulate the clearance of the material in a localized
manner.
Owing to its unique physicochemical properties, un-
modified HAc has been widely used in the field of visco-
surgery, visco-supplementation, and wound healing.
However, the poor mechanical properties of this water-
soluble polymer and its rapid degradation
in vivo
have
precluded many clinical applications. Therefore, in an
attempt to obtain materials that are more mechanically
and chemically robust, a variety of covalent crosslinking
via hydroxyl or carboxyl groups, esterification, and
annealing strategies have been explored to produce in-
soluble HAc hydrogels. For example, HAc-esterified
materials, collectively called ''Hyaff,'' are prepared by
alkylation of the tetrabutylammonium salt of HAc with
an alkyl or benzyl halide in dimethyl formamide solution.
Crosslinked HAc has been prepared using divinyl sul-
fone, 1,4-butanediol diglycidyl ether, GA, WSC, and
a variety of other bifunctional crosslinkers. However, the
crosslinking agents are often cytotoxic small molecules,
and the resulting hydrogels have to be extracted or
washed extensively to remove traces of unreacted re-
agents and by-products.
M and G units (
Fig. 7.2-4
). The length of the M- and
G-blocks and sequential distribution along the polymer
chain varies depending on the source of alginates. These
biopolymers undergo reversible gelation in aqueous so-
lution under mild conditions through interaction with
divalent cations including Ca
2
þ
,Ba
2
þ
, and Sr
2
þ
that
can cooperatively bind between the G-blocks of adjacent
alginate chains creating ionic interchain bridges. This
highly cooperative binding requires more than 20
G-monomers.
Gels can also be formed by covalently crosslinking
alginate with adipic hydrazide and poly(ethylene glycol)
(PEG) using standard CDI chemistry. Ionically cross-
linked alginate hydrogels do not specifically degrade
but undergo slow uncontrolled dissolution. Mass of the
alginate-Ca
2
þ
is lost through ion exchange of calcium
followed by dissolution of individual chains, which re-
sults in loss of mechanical stiffness over time. Alginates
are easily processed into any desired shape with the use
of divalent cations. One possible disadvantage of using
alginates is its low and uncontrollable
in vivo
degradation
rate, mainly due to the sensitivity of the gels towards
calcium chelating compounds (e.g., phosphate, citrate,
and lactate). Several
in vivo
studies have shown large
variations in the degradation rate of calcium-crosslinked
sodium alginates. Hydrolytically degradable form of
alginate and an alginate derivative, polyguluronate,
are oxidized alginate and poly(aldehyde guluronate),
respectively.
Alginate
Alginates are linear polysaccharide derived primarily
from brown seaweed and bacteria. They are block co-
polymers composed of regions of sequential (1-4)-linked
b-
D
-mannuronic acid monomers (M-blocks), regions of
a-
L
-guluronic acid (G-blocks), and regions of interspersed
Chondroitin sulfate
Chondroitin sulfate (CS) is composed of repeating di-
saccharide units of glucuronic acid and
N
-acetylga-
lactosamine with a sulfate group and a carboxyl group on