Biomedical Engineering Reference
In-Depth Information
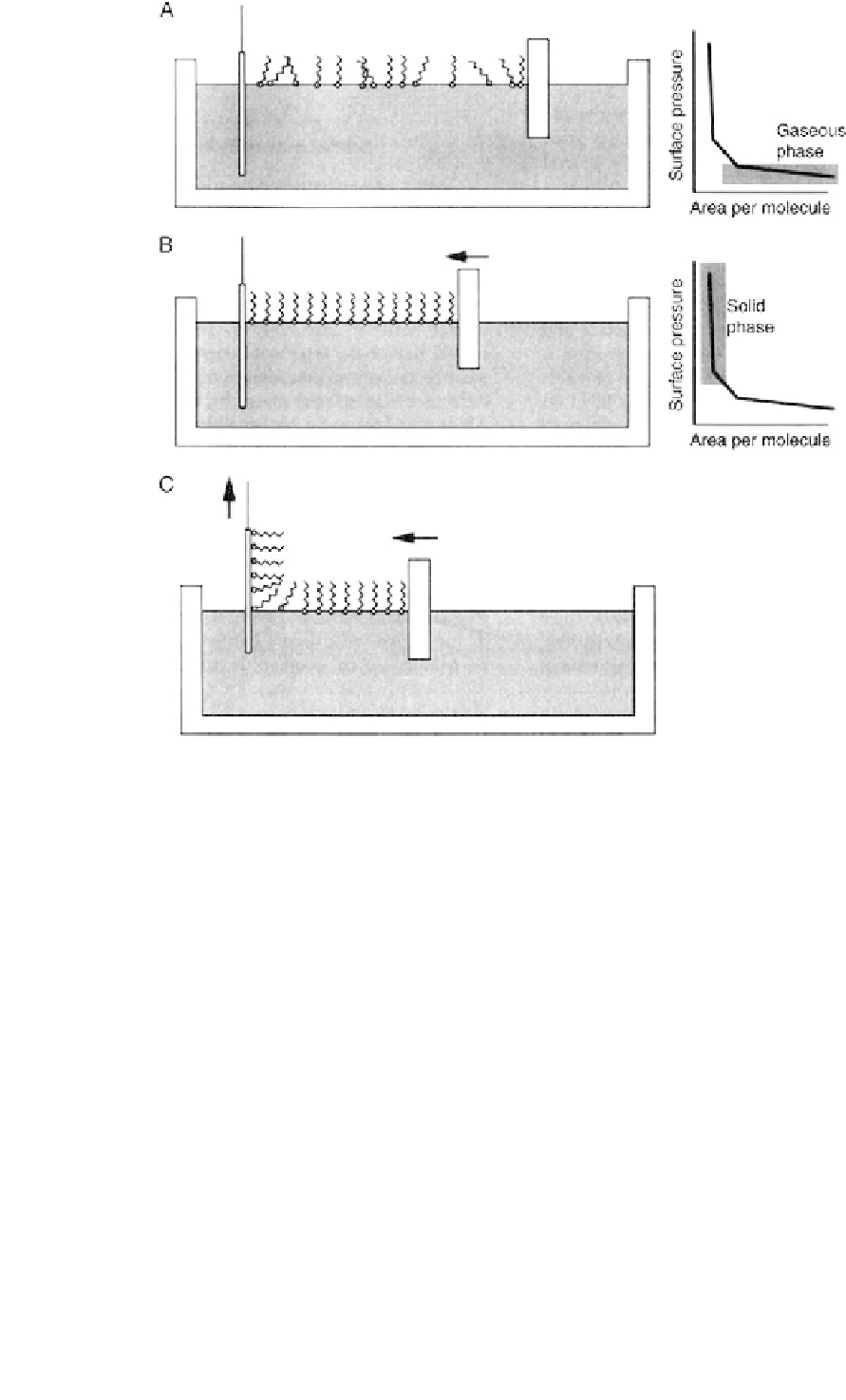
Fig. 3.2.14-6 Deposition of a lipid film onto a glass slide by the LB technique. (A) The lipid film is floated on the water layer. (B) The lipid
film is compressed by a moveable barrier. (C) The vertical glass slide is withdrawn while pressure is maintained on the floating lipid film with
the moveable barrier.
ways SAMs resemble LB films, but there are important
differences, in particular their ease of formation. Exam-
ples of SAM films include
n
-alkyl silanes on hydroxylated
surfaces (silica, glass, alumina), alkane thiols [e.g.,
CH
3
(CH
2
)
n
SH] and disulfides on coinage metals (gold,
silver, copper), amines and alcohols on platinum, car-
boxylic acids on aluminum oxide, and silver and phos-
phates (phosphoric acid or phosphonate groups) on
titanium or tantalum surfaces. Silane SAMs and thiols on
gold are the most commonly used types. Most molecules
that form SAMs have the general characteristics illus-
trated in
Fig. 3.2.14-8
. Two processes are particularly
important for the formation of SAMs (Ulman, 1991):
a moderate to strong adsorption of an anchoring chemical
group to the surface (typically 30-100 kcal/mol), and van
der Waals interaction of the alkyl chains. The bonding to
the substrate (chemisorption) provides a driving force to
fill every site on the surface and to displace contaminants
from
compression to the LB film by the movable barrier in the
trough. Once adsorption sites are filled on the surface,
the chains will be in sufficiently close proximity so that
the weaker van der Waals interactive forces between
chains can exert their influence and lead to a crystalliza-
tion of the alkyl groups. Fewer than nine CH
2
groups do
not provide sufficient interactive force to stabilize the
2D quasicrystal and are difficult to assemble. More
than 24 CH
2
groups have too many options for defects in
the crystal and are also difficult to assemble. Molecules
with lengths between nine and 24 methylene groups
will assemble well. Molecular mobility is an important
consideration in this surface crystal formation process so
that (1) the molecules have sufficient time to maneuver
into position for tight packing of the binding end groups
at
the
surface
and
(2)
the
chains
can
enter
the
quasicrystal.
The advantages of SAMs are their ease of formation,
their chemical stability (often considerably higher than
the surface. This process
is analogous
to the