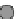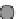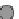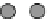Biomedical Engineering Reference
In-Depth Information
Thousands of atoms
may move
Sputtered
atoms
from
substrate
Ti
+
50 KeV
Vacancies
created
Reflected
primary ions
Heating in
surface region
Ti
distribution
with depth
Fig. 3.2.14-5 Some considerations for the ion implantation process.
properties (Picraux and Pope, 1984; Colligon, 1986;
Sioshansi, 1987; Nastasi
etal.,
1996).
Specific examples of biomaterials that have been
surface altered by ion implantation processes are plenti-
ful. Iridium was ion implanted in a Ti-6Al-4V alloy to
improve corrosion resistance (Buchanan
et al.,
1990).
Nitrogen implanted into titanium greatly reduces wear
(Sioshansi, 1987). The ion implantation of boron and
carbon into type 316L stainless steel improves the high
cycle fatigue life of these alloys (Sioshansi, 1987). Silver
ions implanted into polystyrene permit cell attachment
(Tsuji
et al.
, 1998).
multilayer structures can be created. Some compounds
that form organized LB layers are shown in
Fig. 3.2.14-7
.
The advantages of films deposited on surfaces by this
method are their high degree of order and uniformity.
Also, since a wide range of chemical structures can form
LB films, there are many options for incorporating new
chemistries at surfaces. The stability of LB films can be
improved by cross-linking or internally polymerizing the
molecules after film formation, often through double
bonds in the alkyl portion of the chains (Meller
et al.,
1989). A number of research groups have investigated LB
films for biomedical applications (Hayward and Chap-
man, 1984; Bird
et al.
, 1989; Cho
et al.
, 1990; Heens
et al.
, 1991). A unique cross between silane thin films and
LB layers has been developed for biomedical surface
modification (Takahara
et al.
, 2000). Many general re-
views on these surface structures are available (Knobler,
1990; Ulman, 1991).
LB deposition
The (LB) deposition method overcoats a surface with
one or more highly ordered layers of surfactant mole-
cules. Each of the molecules that assemble into this layer
contains a polar ''head'' group and a nonpolar ''tail'' group.
The deposition of an LB film using an LB trough is il-
lustrated schematically in
Fig. 3.2.14-6
. By withdrawing
the vertical plate through the air-water interface, and
then pushing the plate down through the interface,
keeping the surface film at the air-water interface com-
pressed at all times (as illustrated in
Fig. 3.2.14-6
),
SAMs
SAMs are surface films that spontaneously form as
highly ordered structures (two-dimensional crystals) on
specific substrates (Maoz
et al.
, 1988; Ulman, 1990,
1991; Whitesides
et al.
, 1991; Knoll, 1996). In some