Biomedical Engineering Reference
In-Depth Information
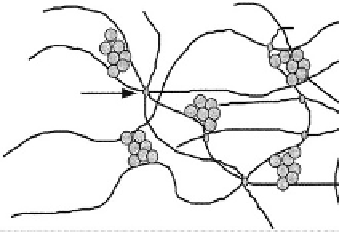
Cross-linking
site
PDMS
Silica
Fig. 3.2.3-2 Silicone elastomer matrix.
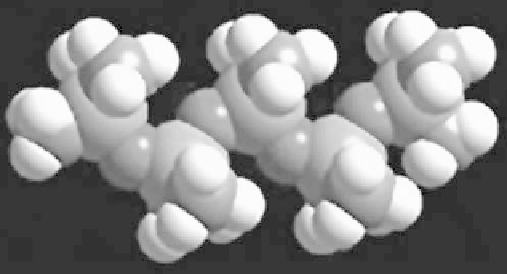
Fig. 3.2.3-3 Three-dimensional representation of dodeca-
methylpentasiloxane, Me
3
SiO-(SiMe
2
O)
3
SiMe
3
or MD
3
M.
(Courtesy S. Grigoras, Dow Corning.)
other medical materials such as PVC, which might con-
tain phthalate additives.
the polymer backbone does not allow a ''selective'' ex-
posure of the most organic or hydrophobic methyl
groups. Chain-to-chain interactions are low, and the
distance between adjacent chains is also greater in sili-
cones. Despite a very polar chain, silicones can be com-
pared to paraffin, with a low critical surface tension of
wetting (Owen, 1981).
The surface activity of silicones is evident in many
ways (Owen, 1981):
The polydimethylsiloxanes have a low surface
tension (20.4 mN/m) and are capable of wetting
most surfaces. With the methyl groups pointing to
the outside, this gives very hydrophobic films and
a surface with good release properties, particularly if
the film is cured after application. Silicone
surface tension is also in the most promising
range considered for biocompatible elastomers
(20-30 mN/m).
Silicones have a critical surface tension of wetting
(24 mN/m) higher than their own surface tension.
This means that silicones are capable of wetting
themselves, which promotes good film formation and
good surface covering.
Silicone organic copolymers can be prepared with
surfactant properties, with the silicone as the
hydrophobic part, e.g., in silicone glycols
copolymers.
The low intermolecular interactions in silicones have
other consequences (Owen, 1981):
Glass transition temperatures are very low, e.g.,
146 K for a polydimethylsiloxane compared to
200 K for polyisobutylene, the analog hydrocarbon.
The presence of a high free volume compared to
hydrocarbons explains the high solubility and high
diffusion coefficient of gas into silicones. Silicones
have a high permeability to oxygen, nitrogen, or
water vapor, even though liquid water is not capable
of wetting a silicone surface. As expected, silicone
compressibility is also high.
Physicochemical properties
Silicon
0
s position just under carbon in the periodic table
led to a belief in the existence of analog compounds
where silicon would replace carbon. Most of these analog
compounds do not exist, or behave very differently.
There are few similarities between Si-X bonds in sili-
cones and C-X bonds (Corey, 1989; Hardman, 1989;
Lane and Burns, 1996; Stark
et al.
, 1982).
Between any given element and Si, bond lengths are
longer than for C with this element. The lower electro-
negativity of silicon (
c
Si
z 1.80,
c
C
z 2.55) leads to
more polar bonds compared to carbon. This bond po-
larity also contributes to strong silicon bonding; for ex-
ample, the SiO bond is highly ionic and has large bond
energy. To some extent, these values explain the stability
of silicones. The SiO bond is highly resistant to homo-
lytic scission. On the other hand, heterolytic scissions are
easy, as demonstrated by the reequilibration reactions
occurring during polymerizations catalyzed by acids or
bases (see earlier discussion).
Silicones exhibit the unusual combination of an in-
organic chain similar to silicates and often associated with
high surface energy, but with side methyl groups that are
very organic and often associated with low surface energy
(Owen, 1981). The Si-O bonds are quite polar and
without protection would lead to strong intermolecular
interactions (Stark
et al.
, 1982). Yet, the methyl groups,
only weakly interacting with each other, shield the main
chain (see
Fig. 3.2.3-3
).
This is made easier by the high flexibility of the silox-
ane chain. Barriers to rotation are low and the siloxane
chain can adopt many configurations. Rotation energy
around a H
2
C-CH
2
bond in PE is 13.8 kJ/mol but only
3.3 kJ/mol around a Me
2
Si-O bond, corresponding to
a nearly free rotation. In general, the siloxane chain
adopts a configuration such that the chain exposes
a maximum number of methyl groups to the outside,
whereas in hydrocarbon polymers, the relative rigidity of