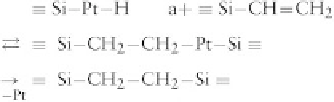Biomedical Engineering Reference
In-Depth Information
shrinkage). Silicones with this cure system are therefore
not suitable for the fabrication of parts with precise
tolerances.
linked matrix. The strength of silicone polymers without
filler is generally unsatisfactory for most applications
(Noll, 1968). Like most other noncrystallizing synthetic
elastomers, the addition of reinforcing fillers reduces
silicone
0
s stickiness, increases its hardness and enhances
its mechanical strength. Fillers might also be employed to
affect other properties; for example, carbon black is
added for electrical conductivity, titanium dioxide im-
proves the dielectric constant, and barium sulfate in-
creases radiopacity. These and other materials are used to
pigment the otherwise colorless elastomer; however, care
must be taken to select only pigments suitable for the
processing temperatures and end-use application.
Generally, the most favorable reinforcement is
obtained using fumed silica, such as Cab-O-Sil, Aerosil,
or Wacker HDK. Fumed silica is produced by the hy-
drolysis of silicon tetrachloride vapor in a hydrogen flame:
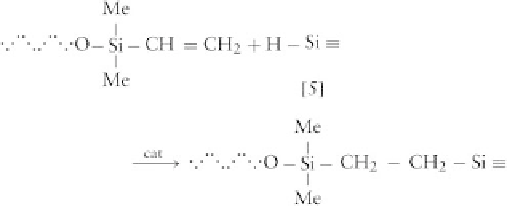
3. Cross-linking by Addition Use of an addition-cure
reaction for cross-linking can eliminate the shrinkage
problem mentioned above. In addition cure, cross-linking
is achieved by reacting vinyl endblocked polymers with
Si-H groups carried by a functional oligomer such as
described above [6]. A few polymers can be bonded to this
functional oligomer [6], as follows (Stark
et al.
, 1982):
1800
C
SiCl
4
þ
2H
2
þ
O
2
!
SiO
2
þ
4HCl
where h represents the remaining valences of the Si
in [6].
The addition occurs mainly on the terminal carbon and
is catalyzed by Pt or Rh metal complexes, preferably
as organometallic compounds to enhance their compati-
bility. The following mechanism has been proposed (oxi-
dative addition of the hSi to the Pt, H transfer to the
double bond, and reductive elimination of the product):
Unlike many naturally occurring forms of crystalline
silica, fumed silica is amorphous. The very small spheroid
silica particles (on the order of 10 nm diameter) fuse
irreversibly while still semimolten, creating aggregates.
When cool, these aggregates become physically entangled
to form agglomerates. Silica produced in this way pos-
sesses remarkably high surface area, 100 to 400 m
2
/g as
measured by the BET method developed by Brunauer,
Emmett, and Teller (Brunauer
et al.,
1938; Noll, 1968;
Cabot Corporation, 1990).
The incorporation of silica filler into silicone polymers
is called ''compounding.'' This is accomplished prior to
cross-linking, by mixing the silica into the silicone poly-
mers on a two-roll mill, in a twin-screw extruder, or in
a Z-blade mixer capable of processing materials with this
rheology.
Reinforcement occurs with polymer adsorption en-
couraged by the silica's large surface area and when hy-
droxyl groups on the filler's surface lead to hydrogen
bonds between the filler and the silicone polymer,
thereby contributing to the production of SRs with high
tensile strength and elongation capability (Lynch, 1978).
The addition of filler increases the polymer
0
s already
high viscosity. Chemical treatment of the silica filler with
silanes further enhances its incorporation in, and rein-
forcement of, the silicone elastomer, resulting in in-
creased material strength and tear resistance (Lane and
Burns, 1996) (
Fig. 3.2.3-2
).
Silicone elastomers for medical applications normally
utilize only fillers of fumed silica, and occasionally ap-
propriate pigments or barium sulfate. Because of their
low glass transition temperature, these compounded and
cured silicone materials are elastomeric at room and body
temperatures without the use of any plasticizers
where, to simplify, other Pt ligands and other Si sub-
stituents are omitted.
There are no by-products with this reaction. Molded
pieces made with silicone using this addition-cure
mechanism are very accurate (no shrinkage). However,
handling these two-part products (i.e., polymer and Pt
catalyst in one component, SiH oligomer in the other)
requires some precautions. The Pt in the complex is
easily bonded to electron-donating substances such as
amine or organosulfur compounds to form stable com-
plexes with these ''poisons,'' rendering the catalyst in-
active and inhibiting the cure.
The preferred cure system can vary by application. For
example, silicone-to-silicone medical adhesives use
acetoxy cure (condensation cross-linking), and platinum
cure (cross-linking by addition) is used for precise sili-
cone parts with no by-products.
4. Elastomer Filler In addition to the silicone poly-
mers described above, the majority of silicone elastomers
incorporate ''filler.'' Besides acting as a material extender,
as the name implies, filler acts to reinforce the cross-
d
unlike