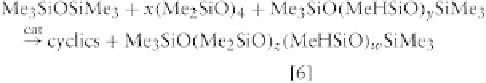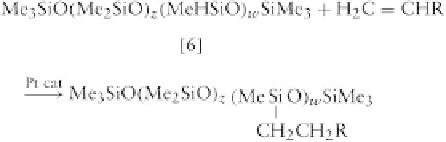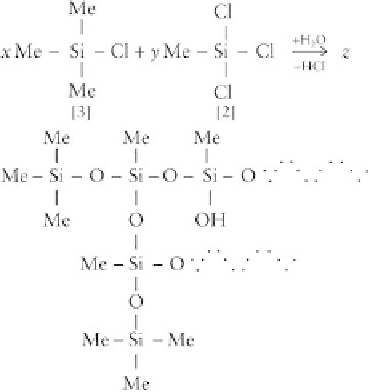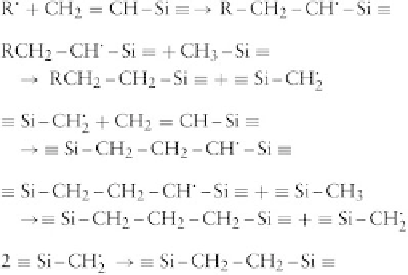Biomedical Engineering Reference
In-Depth Information
existing polymers to obtain a polydimethyl-methylhy-
drogenosiloxane, MD
z
D
H
w
M.
Silicone elastomers
Silicone polymers can be easily transformed into a three-
dimensional network by way of a cross-linking reaction,
which allows the formation of chemical bonds between
adjacent chains. The majority of silicone elastomers are
cross-linked according to one of the following three
reactions.
Additional functional groups can be attached to this
polymer using an addition reaction.
1. Cross-Linking with Radicals Efficient cross-linking
with radicals is only achieved when some vinyl groups are
present on the polymer chains. The following mechanism
has been proposed for the cross-linking reaction associ-
ated with radicals generated from an organic peroxide
(Stark
et al.
, 1982):
The polymers shown are all linear or cyclic, comprising
difunctional units, D. In addition to these, branched
polymers or resins can be prepared if, during hydrolysis,
a certain amount of T or Q units are included, which will
allow molecular expansion, in three or four directions, as
opposed to just two. For example, consider the hydro-
lysis of methyltrichlorosilane in the presence of trime-
thylchlorosilane, which leads to a branched polymer as
shown next:
where h represents two methyl groups and the rest of
the polymer chain.
This reaction has been used for high-consistency sili-
cone rubbers (HCRs) such as those used in extrusion or
injection molding, as well as those that are cross-linked at
elevated temperatures. The peroxide is added before
processing. During cure, some precautions are needed to
avoid the formation of voids by the peroxide
0
s volatile
residues. Postcure may also be necessary to remove these
volatiles, which can catalyze depolymerization at high
temperatures.
2. Cross-Linking by Condensation Although mostly
used in silicone caulks and sealants for the construction
industry and do-it-yourselfer, this method has also
found utility for medical devices as silicone adhesives
facilitating the adherence of materials to silicone elas-
tomers, as an encapsulant and as sealants such as
around the connection of a pacemaker lead to the pulse
generator (
Fig. 3.2.3-1
shows Silastic Medical Adhe-
sive, type A).
These products are ready to apply and require no
mixing. Cross-linking starts when the product is
squeezed from the cartridge or tube and comes into
contact with moisture, typically from humidity in the
ambient air. These materials are formulated from a re-
active polymer prepared from a hydroxy end-blocked
polydimethylsiloxane
The resulting polymer can be described as ((Me
3
SiO
1/2
)
x
(MeSiO
3/2
)
y
or M
x
T
y
, using shorthand notation. The
formation of three silanols on the MeSiCl
3
by hydrolysis
yields a three-dimensional structure or resin, rather than
a linear polymer. The average molecular weight depends
upon the number of M units that come from the trime-
thylchlorosilane, which limits the growth of the resin
molecule. Most of these resins are prepared in a solvent
and usually contain some residual hydroxyl groups. These
could subsequently be used to cross-link the resin and
form a continuous network.
and
a
large
excess
of
methyl-
triacetoxysilane.