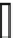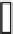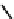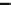Biomedical Engineering Reference
In-Depth Information
CH
3
CH
3
CH
3
C
CH
3
CH
2
CH
2
C
CH
2
C
H
2
C
C
n
O
n
C
O
C
C
OCH
2
CH
2
O
C
O
O
CH
3
C
2
H
5
OH
Poly(methyl-methacrylate)
(PMMA)
Poly(2-hydroxyethyl-
methacrylate) Poly(HEMA)
Ethyleneglycol
dimethacrylate
(EGDM)
CF
2
CH
CF
2
CH
2
CH
2
CH
2
CH
3
Polyethylene (PE)
Polytetrafluoroethylene
(PTFE)
Polypropylene (PP)
O
O
CH
3
(
CH
)
CH
CH
2
C
O
O
C
Si
O
2
2
Cl
CH
3
Polyvinylchloride
(PVC)
Polydimethylsiloxane (PDMS)
(silicone rubber)
Polyethyleneterephthalate
(PET)
CH
2
OH
O
cellulose
OH
OH
O
O
HO
OH
HOCH
2
O
n
+
H
2
N
(CH
2
)
6
NH
2
HO
CO
(CH
2
)
4
CO
OH
adipic acid
hexamethylene
diamine
Ac-OH
Ac-
[
HN- (CH
2
)
6
-NH-CO- (CH
2
)
4
-CO
]
n
- HN - (CH
2
)
6
- NH - Ac
Nylon 6,6
Fig. 3.2.2-5 Homopolymers used as biomaterials.
Condensation polymerization can also result in co-
polymer formation. The properties of the condensation
copolymer depend on three factors: the chemistry of
monomer units; the molecular weight of the polymer
product, which can be controlled by the ratio of one re-
actant to another and by the time of polymerization; and
the final distribution of the molecular weight of the co-
polymer chains. The use of bifunctional monomers gives
rise to linear polymers, while multifunctional monomers
may be used to form covalently cross-linked networks.
Figure 3.2.2-6
shows the reactant monomers and poly-
mer products of some biomedical copolymers.
Postpolymerization cross-linking of addition or con-
densation polymers is also possible. Natural rubber, for
example, consists mostly of linear molecules that can be
cross-linked to a loose network with 1-3% sulfur (vul-
canization) or to a hard rubber with 40-50% sulfur
(
Fig. 3.2.2-3
). In addition, physical, rather than chemical,
cross-linking of polymers can occur in microcrystalline
regions, that are present in nylon (
Fig. 3.2.2-7
A). Al-
ternatively, physical cross-linking can be achieved
through incorporation of ionic groups in the polymer
(
Fig. 3.2.2-7
B). This is used in acrylic acid cement sys-
tems (e.g., for dental cements) where divalent cations