Biomedical Engineering Reference
In-Depth Information
initiation, propagation, and termination to give the final
polymer product. The initiators can be free radicals,
cations, anions, or stereospecific catalysts. The initiator
opens the double bond of the monomer, presenting an-
other ''initiation'' site on the opposite side of the mono-
mer bond for continuing growth. Rapid chain growth
ensues during the propagation step until the reaction is
terminated by reaction with another radical, a solvent
molecule, another polymer molecule, an initiator, or an
added chain transfer agent. PVC, PE, and PMMA are
relevant examples of addition polymers used as bio-
materials. The polymerization of MMA to form PMMA
is shown in
Fig. 3.2.2-2
A.
Condensation polymerization is completely analogous
to condensation reactions of low-molecular-weight mole-
cules. Two monomers react to form a covalent bond, usually
with elimination of a small molecule such as water, hydro-
chloric acid, methanol, or carbon dioxide. Nylon and PET
(
Fig. 3.2.2-2
B) are typical condensation polymers and are
used in fiber or fabric form as biomaterials. The reaction
continues until almost all of one reactant is used up. There
are also polymerizations that resemble the stepwise growth
of condensation polymers, although no small molecule
is eliminated. Polyurethane synthesis bears these charac-
teristics, which is sometimes referred to as polyaddition
or rearrangement polymerization (
Brydson, 1995
).
The choice of polymerization method strongly affects
the polymer obtained. In free radical polymerization,
a type of addition polymerization, the molecular weights
of the polymer chains are difficult to control with pre-
cision. Added chain transfer agents are used to control
the average molecular weights, but molecular weight
distributions are usually broad. In addition, chain transfer
reactions with other polymer molecules can produce
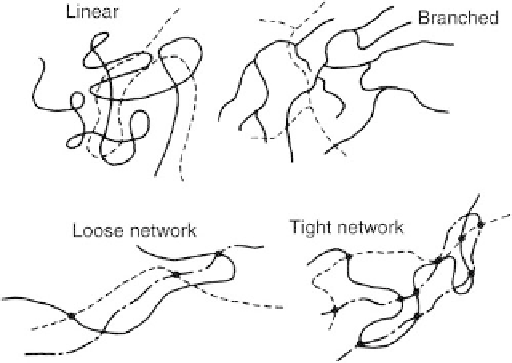
undesirable branched products (
Fig. 3.2.2-3
) that affect
the ultimate properties of the polymeric material. In
contrast, molecular architecture can be controlled very
Fig. 3.2.2-3 Polymer arrangements. (From F. Rodriguez,
Principles of Polymer Systems, Hemisphere Publ., 1982, p. 21,
with permission.)
precisely in anionic polymerization. Regular linear chains
with PI indices close to unity can be obtained. More
recent methods of living free radical polymerizations
called ATRP and RAFT may also yield low PIs.
Polymers produced by addition polymerization can be
homopolymers, i.e., polymers containing only one type of
repeat unit, or copolymers with two or more types of
repeat units. Depending on the reaction conditions and
the reactivity of each monomer type, the copolymers can
be random, alternating, graft, or block copolymers, as
illustrated in
Fig. 3.2.2-4.
Random copolymers exhibit
properties that approximate the weighted average of
those of the two types of monomer units, whereas block
copolymers tend to phase separate into a monomer-A-
rich phase and a monomer-B-rich phase, displaying
properties unique to each of the homopolymers.
Figure 3.2.2-5
shows the repeat units of many of the
homopolymers used in medicine.
AAAAAAAAAAAAAA
B
homopolymer
B
AABABAABBAAABBBA
AAAAAAAAAAAA
random copolymer
graft copolymer
ABABABABABABABAB
AAAABBBBBBAAAAA
alternating copolymer
block copolymer
Fig. 3.2.2-4 Possible structures of polymer chains.