Biomedical Engineering Reference
In-Depth Information
(b)
One factor
1500
Design-Expert® Software
original tabulated scale
GMFI
Design points
1250
X1 = A: Cell concentration
Actual factors
B: Type of fluorescent dye
= Alexa 647
C: Concentration of labeled
detector = 2.5
1000
750
500
0.5e6
1e6
A: Cell concentration
(c)
Interaction
B: Type of fluorescent dye
25
Design-Expert® Software
S/N Ratio
Design points
B1 FITC
20
2
15
B2 Alexa 488
B3 Alexa 647
X1 = A: Cell concentration
X2 = B: Type of fluorescent
dye
10
Actual factor
C: Concentration of labeled
detector = 2.5
5
0.5e6
1e6
A: Cell concentration
FIGURE 10.4
(Continued )
10.3 ASSAY OPTIMIZATION, DEVELOPMENT, AND VALIDATION
Guidelines and consensus publications already exist for immunogenicity method
development, validation, and testing, including antibody [17-23] and neutralizing
antibody assays [1]. These guidelines should be followed and can be applied to any
assay platform used, including flow cytometry. Table 10.1 includes a listing of
recommended studies that should be included in the optimization, development, and
validation stages of cell-based flow cytometric neutralizing antibody assays. Studies
overlap in the different assay phases and are repeated with larger n in the subsequent
assay phase. Performance of a cell-based flow cytometric assay may be affected by
cellular growth conditions, protein expression levels, biological sample matrices, and
critical assay reagents. These parameters should be optimized and tested during the
assay development phase.


































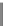












































Search WWH ::

Custom Search