Environmental Engineering Reference
In-Depth Information
3.2 Selected Examples of Soft Matter Systems in Equilibrium
We provide in this section examples of DPD with soft potentials for equilibrium sim-
ulations. When beads are joined through a harmonic potential polymer chains can
easily be simulated, as well as other types of molecules such as surfactants, rheology
modifiers and others (Groot and Warren
1997
). The influence of the characteristics of
the harmonic force forming surfactant molecules on the interfacial tension between
two immiscible liquids has been studied thoroughly (Gama Goicochea et al.
2007
)
and it was shown that the predictions follow closely the experimental trends. Most
applications of DPD have been carried out at constant temperature although there
are plenty of situations of importance for modern research where the influence of
temperature is not sufficiently well understood. One of such cases is the dependence
on temperature of the interfacial tension between liquids. Very recently, an exten-
sion of the DPD model that incorporates the variability of the temperature has been
proposed by Mayoral and Gama Goicochea
2013
. It is based on the addition of the
temperature dependence of the solubility parameters for the pure components in a
mixture, which leads to temperature dependent interaction parameters, (see Eqs.
8
and
9
). Applying the temperature dependent form of DPD to the interfacial tension
between organic solvents and water led to predictions that are in excellent agreement
with experimental results (Mayoral and Gama Goicochea
2013
). Another important
extension of the DPD model is the incorporation of electrostatic interactions through
the method of the Ewald sums. Using distributions of charge instead of point charges
to avoid the formation of artificial ionic pairs, the behavior of poly-electrolytes in
solution under the influence of varying ionic strength has been predicted and com-
pared with similar studies carried out with certain proteins (Alarcón et al.
2013b
).
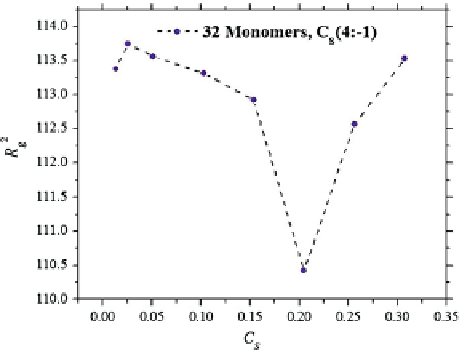
In particular, it has been shown that strongly charged poly-electrolytes in aqueous
solution experience a contraction followed by a re-expansion as a function of the
tetravalent salt content, as shown in Fig.
3
.
Fig. 3
The squared radius of
gyration
R
g
of a
poly-electrolyte made up of
32 equally charged
monomers, in aqueous
solution with varying ionic
strength
C
s
. The latter is
made up of tetravalent ions
of
Na
neutralized with
Cl
ions. The axes are drawn in
reduced DPD units. Adapted
from Alarcón et al. (
2013b
)
Search WWH ::

Custom Search