Environmental Engineering Reference
In-Depth Information
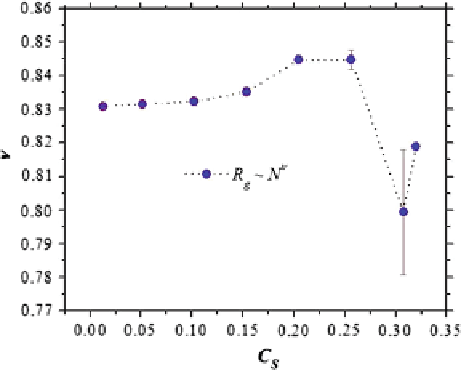
Fig. 4
The scaling exponent
of the radius of gyration as a
function of the
polymerization degree under
a varying concentration of
Na and Cl ions. The
y
-axis is
dimensionless while the
x
-axis is shown in reduced
DPD units. Adapted from
Alarcón et al. (
2013b
)
The contraction-re-expansion behavior quantified through the radius of gyration
R
g
of a model poly-electrolyte shown in Fig.
3
is the result of a complex interplay
between the electrostatic interaction and the excluded volume effect brought about
by the softness of the DPD interaction potential. Moreover, it is by no means an
artifact of the DPD model, as it has been obtained using other interaction potentials
and has also been observed in experiments with proteins in aqueous solution (Hsiao
and Luijten
2006
). In fact, using this electrostatic version of DPD it has been possible
to predict the scaling exponent of the radius of gyration, defined by the well-known
relation
R
g
∼
N
ʽ
where
N
is the polymerization degree and
is the scaling exponent
(Alarcón et al.
2013b
). It is known that it depends on the quality of the solvent in which
the polymer is immersed, and on the dimension of the system. For neutral polymers
in a good solvent it has been shown for some time that
ʽ
588. However, for
poly-electrolytes much less is known. To begin with, it is not even firmly established
if a scaling relation is obeyed and much less what the value of the scaling exponent
should be. Nevertheless, several groups have shown that poly-electrolytes do show
scaling characteristics in their radius of gyration, but their scaling exponent is usually
larger that its counterpart for neutral polymers.
Figure
4
shows the values of the scaling exponent of the radius of gyration for
model linear poly-electrolytes of different polymerization degrees as a function of
the ionic strength. Although the results in Fig.
4
were obtained for simulations in
which all the interaction parameters between the poly-electrolyte and solvent were
kept the same, the values of the scaling exponent are well above the value of 0
ʽ
=
0
.
.
588
for neutral polymer in good solvent. These conditions correspond, in the neutral
case, to the so-called theta solvent. Therefore, the results shown in Fig.
4
demon-
strate that the quality of the solvent can be strongly modified by the electrostatic
interactions.
Search WWH ::

Custom Search