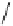Geology Reference
In-Depth Information
If the grain surfaces are in equilibrium with a liquid, then
the above equation modifies to
dry air at −15 °C for 20 min. He demonstrated his etching
technique by replicating the surface with a 1% Formvar
solution and observing the replica with a transmission
electron microscope.
Muguruma
[1961] also made a simi-
lar claim of developing thermal etch pits by allowing a
surface with mirror finish obtained by the Higuchi method
to evaporate slowly for a few minutes at −25 °C and then
replicating the surface with a 0.5% Formvar solution. The
two procedures used by these authors do not act in the way
that was attributed to them. Observation of an ice surface
with a mirror finish under a high‐resolution optical micro-
scope by the second author of this topic (N. K. Sinha) indi-
cated that neither of the procedures for etching in air was
successful in developing pits corresponding to microde-
fects. Both investigators, however, replicated the surface
with a Formvar solution. The author (N. K. Sinha) has
found that pits developed within 20-30s after pouring such
a solution on the surface. In another experiment,
Ketcham
and Hobbs
[1969] measured equilibrium grain boundary
groove angles after maintaining the ice at −3 °C for a period
of 2 days, by making a replica of the grain boundary with
a 5% Formvar solution. It is possible that the replicating
process could have transformed the equilibrium state into
a metastable condition and changed the groove angles.
This possibility was apparent from the work of
Suzuki and
Kuroiwa
[1972] where measurable differences were demon-
strated between the grain boundary groove angles meas-
ured by replica and metallic foil methods.
The foregoing discussion suggests that more informa-
tion can be obtained by the Formvar etching and replicat-
ing process than has been obtained prior to 1975 by
manipulating the conditions of the process, such as the
concentration of the solution, the temperature and
humidity of the surrounding environment, the thickness
of the etchant poured on the surface, and the drying time.
Practical application of these aspects of the dual pro-
cesses of chemical etching/replicating will be discussed
later in Section 6.4.4, but before doing that, it is appropri-
ate to present and discuss a simpler method of investigat-
ing surfaces that did not attract the attention of most ice
researchers. This is the method of thermal etching. The
application of thermal etching of ice surface is simple
and yet extremely powerful. Surprisingly, very little use of
this technique has been made by the ice researchers.
/
12
cos
/
2
(6.5)
LS
gb
LS
where
γ
LS
is the liquid‐solid surface energy and
θ
LS
is liquid‐
solid groove angle.
Measurement of the groove angles provides a method for
estimating grain boundary energies, and this has been
extended to ice also [Hobbs, 1974]. The specific free energy
for the ice‐water interface,
γ
SL
(0.30 erg/mm
2
), is less than
one-third of the ice‐vapor interface,
γ
VS
(1.09 erg/mm
2
) at
0 °C [
Ketcham and Hobbs
, 1969]. There is, therefore, a possi-
bility of using water as the medium for etching. The method
is not practical for most cases, but the possibility was dem-
onstrated by the fact that
Truby
[1955] observed etch pits on
the basal plane of growing ice surface.
Drost‐Hansen
[1967]
considered such pits to be due to dislocations. In this respect,
the observations of
Ketcham and Hobbs
[1968] are very
important. They examined the growing ice‐water interface
for slow growth rates in the range of 0.1-1
μ
m/s. They
observed growth of polycrystalline ice from pure water by
the propagation of spiraling steps with spacing between the
steps in the range of 5-20
μ
m depending on the temperature
gradient in the water. They also noticed the steps to traverse
grain boundaries, but the steps were generally higher than
the depth of the grain boundaries.
Following essentially the traditional practice in metal-
lurgical field, etch pits in ice are obtained by pouring a
thin layer of the etching solution on the surface. In the
case of ice, the Formvar solutions are allowing it to dry
by the evaporation of the solvent under the ambient con-
ditions of a cold room. Due primarily to the high ther-
mal state of ice, the process not only etches the ice surface
but makes a Formvar replica at the same time. This is
a great advantage for using this dual process of etching
and replicating method. This dual use for the etchant,
however, complicates the process of dissociation. Surface
water molecules in ice dissociate in ethylene dichloride.
Formvar dissolved in it acts as the retarding agent, and
the concentration of the solution affects the rate of dis-
sociation. The rate of removal of water molecules from
the ice surface to the solution, however, depends upon
the concentration of water already in the solution. This
concentration changes during etching due to the evapo-
ration of the solvent and this affects the processes of dis-
solution of surface water molecules.
The lack of understanding of this dual etching and rep-
licating process seemed to have misled many workers and
indirectly retarded development and application of this
powerful method. A few examples will be cited here for
clarification.
Truby
[1955] claimed to have developed a
procedure of etching ice surface by a blast of cold and
6.4.3. Thermal Etching of Microtomed Ice Surfaces
Ice is transparent and it is birefringent. Moreover, with
practice, useful thin sections can be prepared quickly by
the hot‐plate technique. These thin sections of ice can be
readily examined with polarized light, which has already
been presented in detail. Consequently, the hot‐plated
thin sections provided sufficient details of petrographic
analysis of salt‐free ice and satisfied the needs for