Biomedical Engineering Reference
In-Depth Information
(a)
Fe(CO)
5
(1) Decomposition
Au
Fe
3
O
4
Au
(2) Oxidation
(b)
(c)
Fe
3
O
4
(111)
0.485 nm
Au(111)
0.24 nm
20 nm
2 nm
(d)
O
EGFRA-NH-CO-PEG(3000)-CO-NH
Fe
3
O
4
Au
-S-PEG(2000)-NH
2
O
(e)
(f)
(iii)
(iii)
(iii)
(iv)
[Fe] in mM 1.24
0.62
0.31
0.16
0.08
0
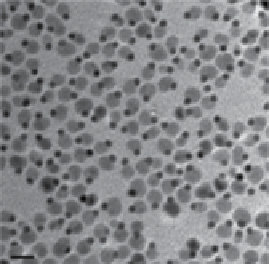
fIgure 2.8
(a) Scheme for the formation of dumbbell-like Fe
3
O
4
/au NPs. (b) TEM and (c)
HRTEM images of the dumbbell Fe
3
O
4
/au NPs. (d) Surface modification of the dumbbell
Fe
3
O
4
/au NPs. (e)
T
2
-weighted MR images of (i) 20 nm Fe
3
O
4
, (ii) 3-20 nm au/Fe
3
O
4
dumb-
bell NPs, (iii) 8-20 nm au/Fe
3
O
4
dumbbell NPs, and (iv) a431 cells labeled with 8-20 nm au/
Fe
3
O
4
dumbbell NPs. (f) Reflection image of a431 cells labeled with 8-20 nm au/Fe
3
O
4
dumbbell NPs. (Reprinted with permission from Ref. [51]. © american chemical Society and
Reprinted with permission from Ref. [52]. © Wiley.)
(RITc)-silane, which make the NPs water soluble and potentially useful for fluores-
cence imaging. dispersed in deionized water, the core-shell Fe
3
O
4
/TaO
x
NPs show a
r
2
value of 81.2 mM
−1
·s
−1
at a 3
T
field. The cT signal intensity constantly increases
with the dose increase.
In vivo
tests on rats bearing mammary adenocarcinoma cells












Search WWH ::

Custom Search