Biomedical Engineering Reference
In-Depth Information
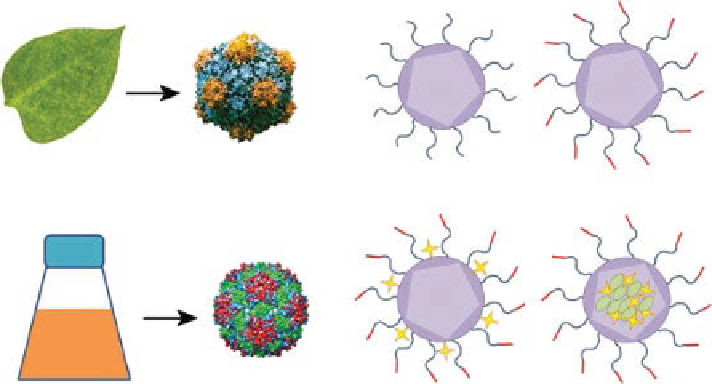
(a)
(b)
Production of VNPs from plants
Covalent display of
PEG on viral capsid
Covalent display of
targeting ligands
Bacteria/
yeast
Covalent display
of imaging agents
Encapsulation of imaging
or therapeutic molecules
Production of VLPs from bacteriophages
figure 14.2
production and chemical engineering of VNps. (a) VNps can be produced in
their natural hosts, such as plants and bacteriophage. Viruslike particles (VLps) can be pro-
duced in a heterologous expression system in bacteria and yeast. (b) After purification, VNps
can be chemically engineered and designed for conjugation or entrapment of targeting ligands,
imaging moieties, and/or therapeutic molecules.
DNA and carboxylate-terminated polyethylene glycol (peG) for the encapsulation of
gold and magnetic iron oxide nanoparticles [33-35].
The structure and physicochemical properties of the protein subunits of VNps
allow them to be biochemically modified or genetically engineered for specific func-
tionalization (Fig. 14.2). A wide variety of bio-orthogonal chemistries have been
developed to allow loading with drug cargos, targeting ligands, and/or imaging moi-
eties such as dyes and clinical contrast agents [15]. examples of genetic modification
include the insertion of amino acids onto the viral capsid to serve as ligation handles
for bioconjugation, the introduction of peptide-based affinity tags for purification,
and the incorporation of targeting ligands for cell-specific delivery [36, 37].
VNps derived from plants and bacteriophages are safe for use in humans, as they
are not the natural hosts for the viruses. plant-derived CpMV, CCMV, and potato
virus X (pVX) nanoparticles as well as Qβ and M13 bacteriophage particles localize
in a wide variety of tissues throughout the body, with accumulation and clearance via
the liver and spleen (organs of the reS) [38-42]. Despite broad biodistribution, no
toxicity or other clinical symptoms have been observed after the administration of
VNps at doses of up to 100 mg/kg body weight in mice [39, 42]. A potential downside
for the use of VNps is immunogenicity of the protein coat. however, the likelihood
of an immunogenic response can be reduced through the conjugation of peG, an
FDA-approved polymer that is nontoxic, neutrally charged, and strongly hydrophilic.
This process, termed peGylation, is a widely used strategy for enhancing solubility
and stability of nanocarriers containing hydrophobic drugs, reducing biospecific
interactions and nonspecific uptake, and increasing plasma circulation time [18, 43].
Search WWH ::

Custom Search