Biomedical Engineering Reference
In-Depth Information
Icosahedral
mammalian virus
Icosahedral plant viruses
Rod-shaped plant viruses
BMV
CCMVCPMV
HCRSV TBSV TYMV
PVX
TMV
Icosahedral bacteriophages
Filamentous phage
AD
M13
Qβ
HK97
P22
T7 S2
figure 14.1
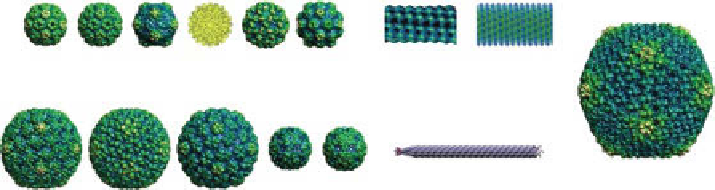
Viral nanoparticles (VNps) currently developed for use in medicine. icosahedral
plant viruses: brome mosaic virus (BMV), cowpea chlorotic mottle virus (CCMV), cowpea
mosaic virus (CpMV), hibiscus chlorotic ringspot virus (hCrSV), tomato bushy stunt virus
(TBSV), and turnip yellow mosaic virus (TYMV). icosahedral bacteriophages: hK97, p22, T7,
MS2, and Qβ. icosahedral mammalian virus: adenovirus (Ad). rod-shaped and filamentous
viruses: potato virus X (pVX), tobacco mosaic virus (TMV), and bacteriophage M13. images
of BMV, CCMV, CpMV, TYMV, hK97, p22, T7, MS2, Qβ, and Ad were generated from the
Viper Database (UrL: http://www.viperdb.scripps.edu/). (The structures of hCrSV, TMV,
and M13 were reproduced with permission from ref. [21]. © elsevier. From refs. [22] and [23].
© National Academy of Sciences).
Numerous VNps have been developed and used in medicine (Fig. 14.1), and each
platform can be tailored to serve distinct functions. plant-based VNps, such as brome
mosaic virus (BMV), cowpea chlorotic mottle virus (CCMV), cowpea mosaic virus
(CpMV), hibiscus chlorotic ringspot virus (hCrSV), tomato bushy stunt virus
(TBSV), and tobacco mosaic virus (TMV), can be produced and purified in large
quantities from plants, while VNps derived from bacteriophages, such as hK97, M13,
MS2, and Qβ, can be produced in gram quantities from
Escherichia coli
bacterium
cultures [19] (Fig. 14.2). VLps derived from eukaryotic viruses can be produced
from baculovirus in insect cells [24] or adenovirus in mammalian cells [25], and
these particles maintain the morphology and cell-penetrating properties of the viruses
from which they were derived [14].
The highly dynamic nature of VNps allows them to undergo transitions that result
in the formation of pores within the protein capsid, permitting access to their interior
cavity for use as a constrained reaction vessel or storage unit [18]. Strategies to encap-
sulate guest molecules into the capsid's interior have been developed. For example,
using a process called gating, the structure of the protein cages of CCMV and red
clover necrotic mosaic virus (rCNMV) can be changed by altering the ph and metal
ion concentration to control the loading or release of cargo molecules from the interior
cavity [26-29]. As VNps are generated through the assembly of protein subunits, cargo
encapsulation can also be achieved through a disassembly and reassembly process for
particles such as CCMV and BMV. Self-assembly naturally occurs through electrostatic
interactions between the protein subunits and rNA. Following nature's paradigm, neg-
atively charged polymers and particles such as polystyrene sulfonate (pSS) for CCMV
and citrate-coated nanoparticles for BMV can be used to initiate capsid reassembly
around foreign cargo [30-32]. Some highly effective facilitators of reassembly include
Search WWH ::

Custom Search