Biomedical Engineering Reference
In-Depth Information
Multiple ligands were sought to provide additional benefits increasing the specificity
of the probe due to many interactions with cell receptors overexpressed at the site of
interest. The first effort to modify the surface of the nanoparticles with antibodies
appeared in the mid-1970s with the pioneering work of gregoriadis. He demon-
strated that
111
In-labeled liposomes carrying “homing probes” such as Igg and desi-
alylated fetuin improved the selectivity of internalization in cell studies [33]. further
progress was made by v. Torchillin's group that decorated liposomes with covalently
conjugated anticanine cardiac myosin antibodies. These targeted liposomes carrying
111
In radiolabels, named “immunoliposomes,” were administered to dogs and were
shown to localize in acute canine myocardial infarctions, thus providing one of the
first examples of target imaging
in vivo
[42]. In addition to antibodies, the surfaces
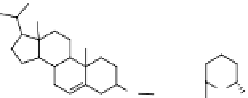
of the nanoparticles were also modified with carbohydrates. exposed 6-aminoman-
nose moieties, through covalent attachments to cholesterol imbedded in the lipid
bilayers (see fig. 1.12), produced a dramatic change in the biodistribution of
nanoparticles labeled with radiolabels and significantly reduced the uptake by the
reS [74].
following liposomes, the development of targeted microbubbles was initiated
during the late 1990s by f. villanueva
et al
. from the University of Pittsburgh. a
40-fold increase in the extent of monoclonal antibody-labeled bubble adhesions to
activated coronary artery endothelium cells compared to nontargeted contrast agents
was observed [75].
Similar to other nanoparticles of that time, early magnetite nanoparticles as a
result of reS sequestration were mostly utilized for imaging of the liver, spleen, and
bone narrow system. Because of reS, these nanoparticles also suffered a short
blood lifetime. Hence, a significant effort over the following several years had been
made to develop new synthetic routes and surface modification techniques to
increase the lifetime and alter biodistribution and pharmacokinetics of iron oxide
nanoparticles [41, 76].
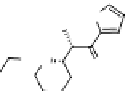
OH
OH
HO
O
OH
3+
In
N
O
O
(CH)
6
CH
2
NH
2
O
NManCholesterol
O
O
O
HN
111
In
3+
nitrilotriacetic
acid
O
N
H
HO
N
O O
O
O
A21387
figure 1.12
Design of targeted liposomes: cholesterol acts as a carrier for the targeting
group,
111
In is complexed by nitrilotriacetic acid, and a21387 acts as a cation ionophore,
allowing these ions to cross cell membranes. The key modification was a sugar derivative
of cholesterol, a standard building block that adds fluidity to the liposome. The presence of
particular surface carbohydrate modifications affected dramatically the stability and tissue
specificity in mice. (Based on ref. [74].)
























































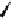

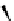
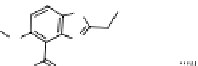

Search WWH ::

Custom Search