Biomedical Engineering Reference
In-Depth Information
replacement of the metal by another one in much higher concentration such as
Fe
3+
, Cu
2+
, or Zn
2+
or by release of the cation of interest into another endogenous
chelator. More than the thermodynamic stability, above all the kinetic inertness must
be high to achieve adequate
in vivo
stability to obviate concerns regarding toxicity.
Kinetic inertness can be determined by competition reaction of the complex in the
presence of other cations such as Zn
2+
, the main competitor in the case of Gd
3+
as
suggested by Laurent
et al
. [17]. The chelating agent must include enough donor
atoms (generally seven or eight nitrogen and negative oxygen atoms are involved),
and the size of the chelate ring should be five for Gd
3+
to form stable complexes [14].
Furthermore, higher preorganized ligands such as macrocycles generally lead to
higher stability than the more flexible open-chain analogs. This has been demon-
strated for Gd
3+
by several comparative studies of cyclic and acyclic chelates in
animals and humans [18].
However, another difficulty that has to be overcome when designing Gd complexes
for MRI is that the relaxivity must not suffer from the gain in stability. Theoretically,
the best relaxivity would be obtained by the octa- or nonahydrated Gd
3+
. The hydration
number,
q
, is a major parameter governing the relaxivity. The
q
value is equal to only
one in each of the approved CAs. As expected, studies with heptacoordinating ligands
such as dTTA or AAZTA (Chart 8.2) leading to complexes with
q
= 2 have shown
that the relaxivity was approximately two to four times higher than CAs with only
one coordinated water molecule [19-21]. Alternatives to polyaminocarboxylic
acid-based ligands have been proposed. The ligands developed in Raymond's group
contain bidentate hydroxypyridinone (HOpO) units leading to hexacoordinated Gd
3+
with two or three bound water molecules (Chart 8.2) [22, 23]. High relaxivities (up to
13 mM
−1
s
−1
at 20 MHz) and high stabilities were observed.
However, hydration number is not the only parameter governing relaxivity. The
residence time of the coordinated molecule,
τ
m
, plays a key role and is influenced by
several features such as the charge of the complex or accessibility to the water
binding site [24]. efforts are made to obtain short
τ
m
close to the optimal value of
N
N
H
O
H
H
N
HN
O
NH
O
-
O
O
H
H
O
O
-
H
O
O
-
O
N
O
-
O
-
HN
Gd
3+
O
O
N
Gd
3+
N
O
O
O
N
Gd
3+
-
O
N
N
H
O
-
O
-
N
O
N
O
O
-
O
-
O
-
H
O
O
O
O
H
H
H
O
O
H
O
N
H
Gd-DTTA
Gd-AAZTA
Gd-TACN-1-Me-3,2-HOPO
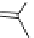
chaRt 8.2
examples of complexes with two or three water molecules bound to the
gadolinium ion.



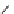

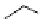


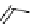
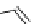
















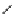

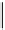

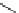

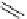
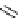




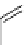
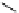
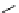

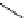
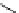
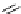



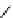

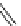


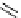



















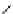
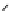
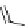
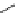


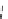

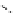

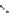






















Search WWH ::

Custom Search