Biomedical Engineering Reference
In-Depth Information
dysprosium (dy
3+
) and holmium (Ho
3+
) have larger magnetic moments than Gd
3+
leading to a higher relaxivity, but this comes with an undesirable shift of the proton
resonance frequency induced by their asymmetric electronic ground state [9].
paramagnetic ions such as Gd
3+
belong to the
T
1
class of agents as they affect
longitudinal relaxation rate more than transverse relaxation rate. superparamagnetic
agents such as large iron oxide particles belong to the
T
2
class of agents.
With an ionic radius of 0.99Å, equivalent to Ca
2+
(1.00Å), Gd
3+
competes with Ca
2+
in biological systems, which makes it highly toxic if used in the free form [10-12]. This
toxicity is why the free metal ion cannot be injected directly to the patient; complexa-
tion with a chelating agent is necessary. several studies have focused on the development
of stable sequestering agents, which are the subject of the next section.
8.2.2 complexation chemistry of Gadolinium: a compromise
between stability and Relaxivity
With its high charge (+3) and its relatively small ionic radius (0.99Å), Gd
3+
belongs
to the hard acids according to the HsAB principle of pearson [13]. Consequently, it
binds strongly to hard bases such as nitrogen or negatively charged oxygen atoms. In
aqueous media, Gd
3+
tends to form eight- or nine-coordinated complexes. For these
reasons, the molecules developed and clinically used today are generally derivatives
of two main structures: the linear triamine dTpA and the 12-membered tetraazam-
acrocycle (cyclen) with five or four carboxylate pending arms, respectively, forming
eight-coordinated complexes with the ninth coordination site available to be occupied
by a water molecule (Chart 8.1). The principles of designing chelating agents for
various kinds of metals, including the lanthanides, have been extensively reviewed
[14-16]. They are generally used for purification purposes (e.g., extraction of heavy
metals from water) or medical applications (for extraction of contaminant metals
in blood, in nuclear medicine, or as CAs in MRI). For medical applications, the
chelating agent must strongly bind the metals of interest to avoid release of the free
ions that are often highly toxic to the biological system (by their radiations or chemical
properties). The challenge is all the more difficult since the injected substances are
highly diluted in the human body, exposing the complex to faster transchelation by
O
O
-
O
O
O
-
N
N
N
O
-
O
O
Gd
3+
N
N
O
-
Gd
3+
H
O
N
N
O
-
O
-
H
O
-
-
O
O
O
O
-
O
O
O
H
H
Gd-DTPA (Magnevist®)
Gd-DOTA (Dotarem®)
chaRt 8.1
examples of approved contrast agents for clinical use.







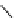
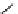


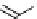

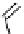
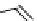
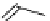

















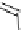
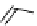













Search WWH ::

Custom Search