Biomedical Engineering Reference
In-Depth Information
0.4
0.3
Large
NPs
Small
NPs
0.2
0.1
Free dye
0
0
10
20
Rotational time (ns/rad)
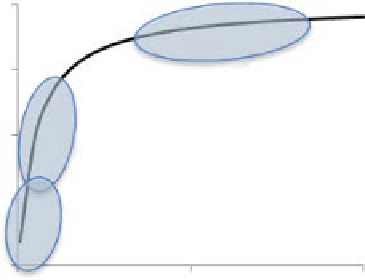
figure 6.23
fluorescence anisotropy as a function of rotational correlation time. larger
and slower nanoparticles show higher anisotropy than free dye and small nanoparticles.
In the ideal case where a nanoparticle has a round shape, the relationship between
r
and
θ
is shown in figure 6.23. free organic fluorophores have a low level of anisotropy
due to their small hydrodynamic volume and, therefore, short rotational correlation
time. fluorophores attached to nanoparticles with a much larger size are expected to
show a higher level of anisotropy. Direct measurements of the rotational time and the
nanoparticles size can be achieved with time-resolved fluorescence anisotropy [81].
Rotational times as low as 1.4 µs and particles with just few nanometers in diameter
can be reliably measured. The drawback with nanoparticle measurements, especially
with larger particles greater than 50 nm, is that the anisotropy value is sensitive to the
excitation light that scatters off the nanoparticle sample. The scattering can signifi-
cantly increase the measured anisotropy value, sometimes reaching a unity (
r
~ 1). To
avoid errors, anisotropy is measured with the wavelength of emission set far from the
wavelength of excitation (up to 100 nm).
Sahoo
et al
. [87] utilized fluorescence anisotropy to confirm the labeling of
magnetic nanoparticles with rhodamine 110. free dye in water exhibited almost no
fluorescence anisotropy (
r
~ 0), but it increased up to 0.4 when the dye was bound to
the nanoparticles. The mobility of fluorophores in the liposomal bilayers provided
information regarding the fluidity of the lipid bilayers [88]. fluorescence anisotropy
has been also utilized to establish successful incorporation of an NIR fluorescent dye
into a nanoparticle [40] and has validated the attachment of nanoparticles to fluoro-
phore-labeled DNa [89]. anisotropy values of quantum dots are rarely measured
because quantum dots usually have a spherical symmetry that results in anisotropy
values close to zero. Indeed, fluorescence anisotropy values for a variety of quantum
dots from 500 to 1100 nm made from different materials and in variety of solvents are
slightly above zero (M.Y. Berezin, personal communication). a deviation from the
zero value of anisotropy from quantum dots has been attributed to a change in
quantum dots' shape [90] and can be utilized to assess the breaking of the electronic
band symmetry.
Search WWH ::

Custom Search