Chemistry Reference
In-Depth Information
The physical characteristics of electrospun nanofi bers such as fi ber diameter de-
pend on various parameters which are mainly divided into three categories: solution
properties (solution viscosity, solution concentration, polymer molecular weight, and
surface tension), processing conditions (applied voltage, volume fl ow rate, spinning
distance, and needle diameter), and ambient conditions (temperature, humidity, and at-
mosphere pressure) [9]. Numerous applications require nanofi bers with desired prop-
erties suggesting the importance of the process control. It does not come true unless
having a comprehensive outlook of the process and quantitative study of the effects of
governing parameters. In this context, Sukigara et al. [10] were assessed the effect of
concentration on diameter of electrospun nanofi bers.
Beside physical characteristics, medical scientists showed a remarkable attention
to biocompatibility and biodegradability of nanofi bers made of biopolymers such as
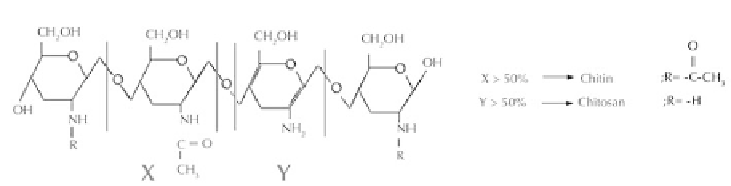
collagen [11], fi brogen [12], gelatin [13], silk [14], chitin [15], and chitosan (CHT)
[16]. Chitin is the second abundant natural polymer in the world and CHT (poly-(1-4)-
2-amino-2-deoxy-β-D-glucose) is the deacetylated product of chitin [17]. CHT is well
known for its biocompatible and biodegradable properties [18].
Scheme 5.1.
Chemical structures of chitin and chitosan biopolymers.
The CHT is insoluble in water, alkali, and most mineral acidic systems. How-
ever, though its solubility in inorganic acids is quite limited, CHT is in fact soluble
in organic acids, such as dilute aqueous acetic, formic, and lactic acids. CHT also has
free amino groups, which make it a positively charged polyelectrolyte. This prop-
erty makes CHT solutions highly viscous and complicates its electrospinning [19].
Furthermore, the formation of strong hydrogen bonds in a 3-D network prevents the
movement of polymeric chains exposed to the electrical fi eld [20].
Different strategies were used for bringing CHT in nanofi ber form. The three top
most abundant techniques includes blending of favorite polymers for electrospinning
process with CHT matrix [21-22], alkali treatment of CHT backbone to improve
electro spinnability through reducing viscosity [23] and employment of concentrated
organic acid solution to produce nanofi bers by decreasing of surface tension [24].
Electrospinning of Polyethylene oxide (PEO)/CHT [21]
and polyvinyl alcohol (PVA)/
CHT [22] blended nanofi ber are two recent studies based on fi rst strategy. In second
protocol, the molecular weight of CHT decreases through alkali treatment. Solutions
of the treated CHT in aqueous 70-90% acetic acid produce nanofi bers with appropri-
ate quality and processing stability [23].
















Search WWH ::

Custom Search