Biomedical Engineering Reference
In-Depth Information
a
NH
3
+
+
H
3
N
b2
b1
I
II
II
IV
+
+
+
+
123
4
5
6
123
4
5
6
123
4
5
6
123
4
5
6
+
+
+
+
-
O
2
C
h
CO
2
-
P
Pore
P
+
H
3
N
CO
2
-
Inactivation
loop
Voltage
Sensing
P
P
P
P
C-terminus
Modulation
= Ion Selectivity
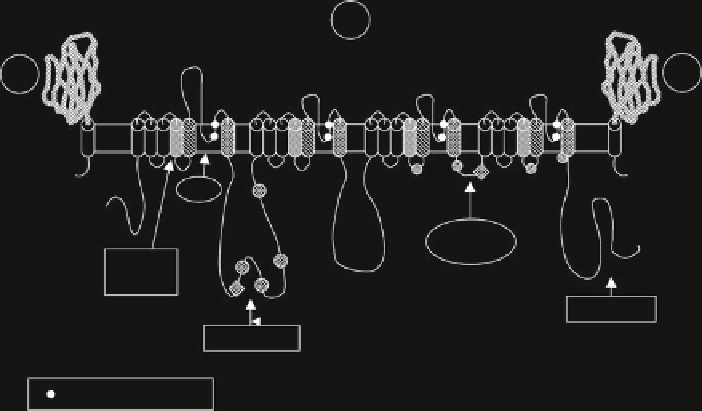
Fig. 1 VGSC subunit structure: VGSC
a
-subunit consists of four domains, I, II, III and IV, each
one developed through six
a
-helical transmembrane segments, S1-S6 (cylinders 1-6). S4
segments in each domain contain positively charged amino acids and function as voltage sensors.
Black solid circles
represent negatively charged amino acids forming the ion selectivity loop. The
intracellular loop between domain III (S6) and domain IV (S1) closes the cytoplasmic end of the
channel pore leading to fast inactivation. P in
circles
and
diamonds
represents phosphorylation
sites by kinases. Each VGSC
-subunit displays a large extracellular immunoglobuline-like N
terminus. Reprinted with permission from [
3
]. Copyright 2000 Cell press
b
have similar primary structure to neural cell adhesion molecules (CAMs), whereas
they share no homology with their counterparts of calcium and potassium channels.
b
1and
b
3 covalently bind to the
a
-subunit by a disulfide bridge; conversely,
b
2and
b
4 associate with the
a
-subunit only weakly.
2.2 VGSC Functions
The primary role of VGSCs is to trigger the rising phase of action potential in most
excitable cells in mammals [
3
,
14
]. The transition from a resting state (Fig.
2a
),
when the channel is closed, to an active state (Fig.
2b
), when the channel is open
and Na
+
ions can enter the pore, is called gating [
2
]. In the case of VGSCs, gating is
governed by voltage signals. The highly conserved S4 transmembrane segments
contain a motif of three positively charged amino acids (Fig.
1
), which are exposed
to the intracellular surface in the resting state and move outwardly in response to
depolarization. Details on the S4 movement toward the outer face of the membrane
are currently controversial. Nonetheless, it is commonly accepted that consequen-
tial conformational changes cause the pore to open [
15
,
16
]. Within a few
milliseconds after the channel opening, an inward Na
+
current is generated and