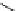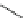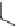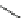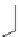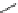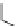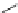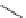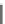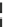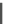Biomedical Engineering Reference
In-Depth Information
R
1
R
1
R
1
R
1
COOR
3
R
2
COOR
3
R
2
COOR
3
R
2
COOR
3
N
N
N
N
N
H
CH
3
X
R
2
X
O
N
H
CH
3
N
H
CH
3
X
N
H
CH
3
X = S, O, N
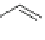
Fig. 8 Dihydropyrimidine analogues
conformational requirements for calcium-channel antagonist-agonist modulation
activity are still not well defined [
40
,
55
]. To study in detail the structure-activity
relationships of unsymmetrical molecules and to find the effects of absolute stereo-
chemistry on biological activity, Atwal et al. [
56
-
59
] studied four different series
of dihydropyrimidine analogs (Fig.
8
). The latter were found to highly imitate
dihydropyridines and to adopt in solid state molecular conformation similar to the
reported conformation of dihydropyridines.
Gupta in a QSAR analysis [
60
] showed that almost all those characteristics
that are essential for the activity of 1,4-dihydropyridines are also essential for
the activity of dihydropyrimidine analogs: conformation of the molecule, the
relative orientation of the aryl ring with respect to the pyrimidine ring, and some
substituents capable of forming the hydrogen bonds with the receptor but less bulky
in nature, and high molar refractivity of the molecule.
The following structural features are characterized as prerequisite for the
calcium ions channel-blocking activity:
(a) The nature and position of C-4-aryl ring substituents: optimizes activity. Phenyl
group is preferred. The pseudoaxial conformation of C-4 aryl ring is also
important [
61
-
63
].
(b) The 1, 4-dihydropyridine ring is essential for activity because it is necessary
for hydrogen bonding. Substitution at the N-1 position or the use of oxidized
(piperidine) or reduced (pyridine) ring systems greatly diminish activity [
61
].
Nifedipine and related analogs have been shown to exist in a boat conformation
[
64
]. Protonation of the dihydropyridine ring nitrogen results in a slight devia-
tion of the boat-like conformer of the protonated dihydropyridines from the
planarity producing a twist-like conformation [
65
].
(c) C-3 and C-5 substituents modulate activity and tissue selectivity [
43
,
63
].
Asymmetrical substituents in C-3 and C-5 alter the activity [
48
]. The electronic
features of the oxygen of the carboxyl ester group influenced biological activity.
Carbonyl oxygen participates in hydrogen bonding with the receptor [
66
].
X-ray structural investigations indicate that at least one ester must be in the
cis
arrangement, which is necessary for hydrogen bonding to the receptor
[
54
]. Molecular orbital conformational calculations suggest that both carboxy
groups are preferentially oriented in a plane that intersects the plane of the
dihydropyridine ring with an angle of between 308 and 608 [
66
]. Based on