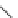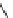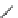Biomedical Engineering Reference
In-Depth Information
other calcium blockers. They also show a minor negative inotropic effect, atrioven-
tricular blockade or neurohormonal activation [
36
-
38
], which often limit their
therapeutic use. They do not show any significant direct effect on the heart. All
seem effective in the clinical situation and they are well tolerated. Their oral
bioavailability is low. Their in vivo metabolism after oral administration is due to
oxidation of the 1, 4-dihydropyridine ring by the cytochrome P450 3A4 isoform
to an aromatic pyridine ring and to oxidative cleavage of the carboxylic esters. The
derivatives are insoluble in water and light sensitive. For the most lipophilic
analogs, the side effects (such as ankle edema) seem less pronounced, most likely
due to their slow onset of activity.
Structure Activity-Relationships: Essential Structural Features and
Physicochemical Properties
Glossmann et al. [
39
] reported that dihydropyridines containing 3, 5-dicarboxylic
acid esters might exhibit a spectrum of activity toward the calcium channel some-
where between the extremes of antagonism and agonism. Attempts were made
to differentiate in the mechanisms of their agonist and antagonist activities.
H
oltje and Marrer [
40
] using quantum mechanical calculations demonstrated
that the molecular potential of a single receptor site was reduced by interaction
with calcium channel activators and increased by interaction with calcium channel
blockers. Matowe et al. [
41
] described the pharmacological properties of AK-2-38
(Fig.
4
), a C-4 2-pyridinyl analog that exhibited twice the potency of nifedipine
on smooth muscle and partial agonism on cardiac muscle. Compounds with the
above properties are known as “dual cardioselective calcium-channel agonist-
smooth muscle selective calcium-channel antagonists” or “third-generation
dihydropyridines” [
42
] (Fig.
4
).
Structure-activity relationship (SAR) studies of dual-acting agents revealed
that although C-4 2-pyridinyl isomer acts as a dual agent, the 3-pyridinyl and
4-pyridinyl isomers are calcium-channel agonists on both heart and smooth muscle
[
43
]. From the above, it is obvious that the position of the pyridinyl nitrogen-free
electron pair and/or charge distribution on the pyridinyl might be important
parameters of calcium-channel agonist-antagonist modulatory effects [
44
]. In
addition, dihydropyridines possessing a C-4-appropriate thienyl isomeric substitu-
ent also exhibit desirable calcium-modulating effects [
45
].
€
H
3
C
N
O
H
3
C
O
CH
3
O
O
N
H
H
3
C
CH
3
Fig. 4 Structure of AK-2-38