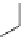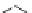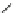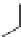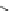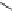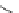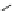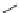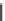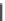Biomedical Engineering Reference
In-Depth Information
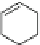
Fig. 2 General structure
of 1,4-dihydropyridines
R
R''OOC
COOR'
N
H
H
3
C
CH
3
Cl
N
O
Cl
N
H
3
COOC
COOCH
2
CH
3
H
3
COOC
COOCH(CH
3
)
2
N
H
Felodipine
H
3
C
CH
3
N
H
Isradipine
H
3
C
CH
3
NO
2
O
2
N
H
3
C(H
2
C)
2
OOC
COOCH(CH
3
)
2
H
3
COOC
COOCH(CH
3
)
2
N
H
H
3
C
CH
3
N
H
H
3
C
CH
3
Nimodipine (
S
)
Nisoldipine
NO
2
Cl
H
3
COOC
COOCH
2
CH
3
H
3
COOC
COOC(CH
3
)
2
CH
2
N(CH
3
)CH
2
CH
2
CH(Ph)
2
N
H
O
NH
2
N
H
H
3
C
CH
3
Amlodipine
Lercanidipine
NO
2
COOC(CH
3
)
3
O
H
3
CH
2
COOC
COOCH
2
CH
3
N
H
3
COOC
N
O
N
H
H
3
C
CH
3
N
H
H
3
C
CH
3
Manidipine
Lacidipine
Fig. 3 Analogs of nifedipine
Nilvadipine (Nivadil), Nimodipine (Nimotop), Nisoldipine (Baymycard, Sular,
Syscor), Nitrendipine (Cardif, Nitrepin, Baylotensin), Pranidipine (Acalas).
Nifedipine. Nifedipine is the lead compound, which was first successfully
introduced for the treatment of coronary angina in Germany at the beginning of
1975. Major disadvantage of its use is the short plasma half-life. The consequence
is multiple daily administrations to achieve blood pressure control (Fig.
3
).