Biomedical Engineering Reference
In-Depth Information
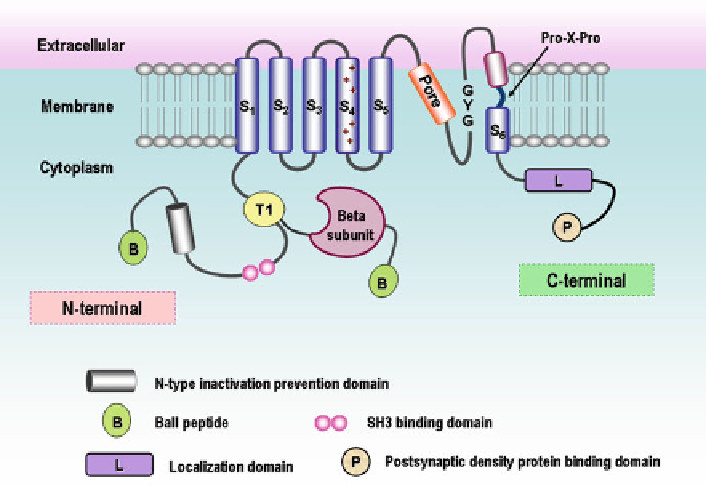
Fig. 1 Schematic illustration of
Shake
family
K
v
channels
3 The Chemical Structures and Structure-Activity
Relationships (SARs) of Potassium Channel Blockers
3.1 Typical I
Kr
(K
v
11.1) Blockers
Ever since Quinidine was isolated from
Cinchona
bark and subsequently identified
as effective agent for cardiac arrhythmia in the beginning of the twentieth century,
antiarrhythmic drugs have been developed for over a hundred years. According to
the Vaughan-William Scheme [
17
], potassium channel blockers belong to Class III
antiarrhythmic agents, which mainly blocked the rapid delayed rectifier potassium
channel currents (
I
Kr
, encoded by
K
v
11.1
gene which is usually called
h
ERG)
[
5
,
18
]. The electrophysiological properties of typical potassium channel blockers
such as Propafenone [
19
], Flecainide [
20
], Sotalol [
21
], Dofetilide, and Ibutilide
[
22
] are the prolongation of action potential duration (APD) and effective refractory
period (ERP). However, the blockade of
I
Kr
would produce negative feedback on
the ventricular repolarizations, which is widely considered as a critical risk factor of
torsades de pointes (TdP) [
23
]. Therefore, clinical applications of typical Class III
antiarrhythmic agents have decreased over the past decade because of its side
effects called “proarrhythmia,” which created more serious rhythm disorders than
being treated.