Biomedical Engineering Reference
In-Depth Information
2.3.4. Lignin
Lignin is a heteropolymer composed primarily of methoxylated phenylpropylene alcohol
monomeric units interconnected by a variety of stable carbon
e
carbon and carbon
e
oxygen
e
carbon (ethers and esters) linkages (
Dence and Lin, 1992
). Structurally, lignin is
a three-dimensional macromolecule. While the lignin of gymnosperms (also called soft-
woods, conifers, needle trees or evergreens) is primarily an enzyme-mediated dehydrogen-
ative polymerization product of coniferyl alcohol (
Fengel and Wegener, 1989
), the lignin of
angiosperms (also hardwoods, deciduous or broad-leaved trees, and grasses) is derived
primarily from a mixture of coniferyl and sinapyl alcohols.
Figure 2.27
shows the three cin-
namyl alcohols, the lignin precursors. The oxygen to carbon ratio in the lignin precursors
(
Fig. 2.27
) is less than 4/11. One can infer that lignin is much less oxygenated than carbohy-
drates, where approximately each carbon atom is accompanied by one oxygen atom as
shown in the previous subsections. The aromatic ring structure also provides excellent func-
tional chemical sources when depolymerized.
The phenylpropane units in lignin are linked together by different bonds, as shown in
Fig. 2.28
. The
b
-O-4 interunit linkages are the most abundant in lignin, estimated to be as
high as 50% in softwoods and almost 60% in hardwoods (
Sj¨ str¨m, 1993
). More than two-
thirds of the phenylpropane units are linked by ether bonds, the rest by carbon
e
carbon
bonds.
Lignins can be divided into several classes according to their structural elements.
Guaiacyl
e
syringyl lignin (GS-lignin) found in hardwoods is a copolymer of coniferyl
and synapyl alcohols (precursors of G- and S-units, respectively) with the G/S ratio
varying from 4:1 to 1:2. Guaiacyl lignin (G-lignin), occurring in almost all softwoods, is
largely a polymerization product of coniferyl alcohol. Different contributions of p-hydrox-
ycinnamyl alcohols in the biosynthesis of hardwood and softwood lignins causes signifi-
cant differences in their structure, including the contents of different types of bonds and
main functional groups, for example, methoxyl (OMe), phenolic hydroxyl (PhOH),
aliphatic hydroxyl, carbonyl, and carboxyl groups. Softwood lignin is more condensed
than hardwood lignin due to the difference in substitution of lignin precursors
(substituted C
3
and unsubstituted C
5
in coniferyl alcohol versus substituted C
3
and C
5
in synapyl alcohol). Significant differences observed between the hardwood and softwood
lignin structure indicate different physical and chemical properties of these two lignin
types.
OCH
3
OCH
3
2
3
1
4
HO
HO
HO
OH
OH
OH
5
6
OCH
3
(a)
trans
-sinapyl alcohol,
(b)
trans
-coniferyl alcohol,
(c)
trans
-coniferyl alcohol,
syringyl unit (S)
guaiacyl unit (G)
p-hydroxyphenyl unit (H)
FIGURE 2.27
The precursors of lignin (Al´n R. Structure and Chemical Composition of Wood. In: Glullichsen J.,
Paulapuro H., editors, Forest Products Chemistry. Jyv¨skyl¨ .Finland Oy 2000).





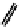

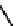
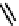

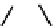
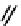
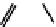
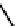
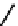
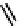





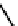

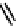

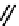
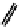
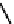
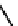














































Search WWH ::

Custom Search