Biomedical Engineering Reference
In-Depth Information
O
O
O
O
O
O
H
H
O
O
n = 6-10
Co Enzyme Ubiquinone:
CoQ
n
n = 6-10
The pyruvate formed in the glycolysis can be oxidized completely to carbon dioxide
and water in the TCA cycle, which is entered via acetyl-CoA. Here, one mole of GTP,
an “energy package” equivalent to ATP, and five reduced cofactor molecules are formed
for each pyruvate molecule. Four of these are NADH, the fifth is FADH
2
. A prerequisite
for the complete conversion of pyruvate in the TCA cycle is that NAD
þ
and FAD can
be regenerated from NADH and FADH
2
. This is done in the respiratory chain (for details,
see
Section 10.7.4
), an oxidative process involving free oxygen and therefore operable only
in aerobic organisms. In the respiratory chain, electrons are passed from NADH to a co-
enzyme called ubiquinone (CoQ or CoQ
n
with n being the number of isoprene units) by
NADH dehydrogenase. They are carried on from CoQ
n
through a sequence of cyto-
chromes (proteins containing a heme group) and are finally donated to oxygen, forming
water.
The net ATP yield in glycolysis is 2 mol ATP/glucose under anaerobic conditions. Pyru-
vate produced in the EMP pathway transfers its reducing power to NAD
þ
via the TCA cycle.
Glycolysis takes place in cytoplasm, whereas the site for the TCA cycle is the matrix of mito-
chondria in eukaryotes. In prokaryotes, these reactions are associated with membrane-bound
enzymes. Entry into the TCA cycle is provided by the acylation of coenzyme-A by pyruvate.
Pyruvate
dehydrogenase
NAD
þ
þ
H
þ
pyruvate
þ
CoA-SH
!
acetyl CoA
þ
CO
2
þ
NADH
þ
(10.39)
Figure 10.23
shows the structure of CoA or CoA-SH and acetyl-CoA or CoA-S-Ac.
Acetyl-CoA is transferred through mitochondrial membrane at the expense of the conver-
sion of the two NADHs produced in glycolysis to 2 FADH
2
. Acetyl-CoA is a key interme-
diate in the metabolism of amino acids and fatty acids.
Figure 10.24
shows the molecular
structures of FAD and FADH
2
. FAD is an aromatic ring (flavin group) system, whereas
FADH
2
is not. This means that FADH
2
is significantly higher in energy, without the stabi-
lization that aromatic structure provides. FADH
2
is an energy-carrying molecule, because,
if it is oxidized, it will regain aromaticity and release all the energy represented by this
stabilization.
A schematic of reactions in a TCA cycle is presented in
Fig. 10.22
. Condensation of
acetyl-CoA with oxaloacetic acid results in citric acid, which is further converted to isoci-
tric acid and then to
-KGA is decar-
boxylated and oxidized to succinic acid (SA), which is further oxidized to fumaric acid
(FA). Hydration of fumaric acid to malic acid (MA) and oxidation of malic acid to
a
-ketoglutaric acid (
a
-KGA) with a release of CO
2
.
a

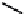




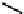



















Search WWH ::

Custom Search