Biology Reference
In-Depth Information
7.4
Curli Biogenesis: Mechanism, Kinetics, and
Fibril Structure
7.4.1
Evidence to Being Naturally Occurring Amyloids
Curli fibres satisfy several mechanistic, structural, and tinctorial
amyloid related features (Fig. 7.2). Curli fibres are insoluble in sodium
dodecyl sulfate (SDS)
31
and resistant to protease digestion.
30
In vivo
assembly depends on interaction with a nucleator protein (CsgB) to
form amyloid-like fibres.
7
Similar to other amyloidogenic proteins,
curli (and curli expressing bacteria) bind the dye Congo red (CR)
and exhibit green birefringence as viewed by cross-polarization
microscopy.
6
Curli were confirmed as naturally occurring amyloid
fibres when they were shown to exhibit a red shift upon CR binding,
to produce a significant fluorescence signal typical to amyloids upon
binding to thioflavin T (ThT) dye, and to display spontaneous self-
assembly by the purified monomers
in vitro
into amyloid-like fibrils.
These fibres were substantially rich in
-sheet content according to
electron microscopy and circular dichroism analysis.
β
4
(a)
(b)
A
A
B
B
(c)
C
C
(d)
D
D
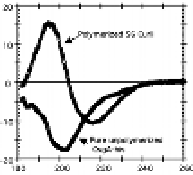
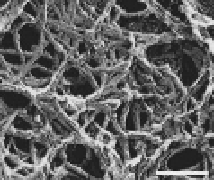
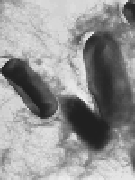
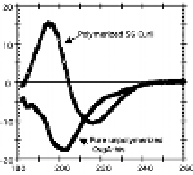
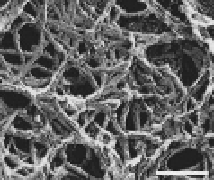
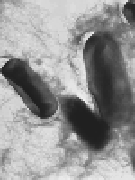
Figure 7.2
Amyloidal characteristics of curli. Transmission electron
microscopy (TEM) micrograph (a) and of high-resolution
deep-etch EM micrographs (b) of
MC4100 expressing
curli (bars: 500 nm and 60 nm, respectively). (c) Congo red
dye binding by curliated (left) and non-curliated (right)
E
.
coli
E
.
coli
MC4100. (d) CD spectrum of curli purified from MC4100
compared with that of soluble unpolymerized CsgA-his,
indicating transition from random-coil to β-sheet rich
structure. (b and d) Reprinted from Chapman
et
al
.,
Science
,
295
(2005) with permission from AAAS. See also Colour Insert.












Search WWH ::

Custom Search