Biomedical Engineering Reference
In-Depth Information
cytotoxicity of ethyl, 2-octyl,
n
-octyl, and ethylhexyl cyanoacrylates by using
different polymerization procedures. They showed that the cyanoacrylate pow-
ders degraded faster than the films and that the ECN gave the faster degradation
and higher level of cytotoxicity (mainly due to formaldehyde evolution).
Considering these previous findings in the existing literature, the aim of this
study was to compare the physical-chemical properties of several cyanoacrylate
monomers and polymers having linear alkyl hydrocarbon chains with different
length. In this study the preliminary experimental results obtained up to now in our
laboratory are reported as the study is currently under development.
5.2
Experimental
5.2.1
Materials
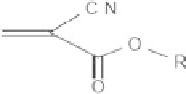
Three cyanoacrylate monomers were used in this study (Fig.
5.1
): ECN (R=CH
2
-CH
3
),
BCN (R=(CH
2
)
3
-CH
3
), and (OCN (R=(CH
2
)
7
-CH
3
).
The ECN monomer was prepared in our laboratory. In the first step (Fig.
5.2
)
polyethyl cyanoacrylate was obtained by reacting ethyl cyanoacetate and formalde-
hyde (both CP grade and provided by Aldrich, Barcelona, Spain) in the presence of
a base catalyst—i.e., piperidinium chloride (Knoevenagel reaction). The catalyst
produced hydrogen abstraction in a position to the carboxyl group in the ethyl
cyanoacetate, and the resulting carbanion was added to formaldehyde, giving the
condensation product. The monomer was produced by depolymerization of the
polyethyl cyanoacrylate by direct heating in a burner [
18
] . The resulting ECN
monomer was further purified through low-pressure distillation. The ECN obtained
had an Ubbelohde viscosity of 2.21 cStokes.
Both the
n
-butyl and
n
-octyl cyanoacrylate monomers used in this study were
commercial products. BCN monomer was
Vetbond
®
manufactured by 3M (St. Paul,
Minnesota, USA) and contains hydroquinone stabilizer and blue dye. OCN was
Dermabond
®
manufactured by Ethicon (Somerville, New Jersey, USA) and con-
tains thickening agent, stabilizer, and violet dye.
R = CH
2
-CH
3
ethyl cyanoacrylate
R = (CH
2
)
3
-CH
3
n-butyl cyanoacrylate
R = (CH
2
)
7
-CH
3
n-octyl cyanoacrylate
Fig. 5.1
Cyanoacrylate
monomers used in this study
Search WWH ::

Custom Search