Environmental Engineering Reference
In-Depth Information
the intraparticle diffusion rate of the dye molecules into the activated car-
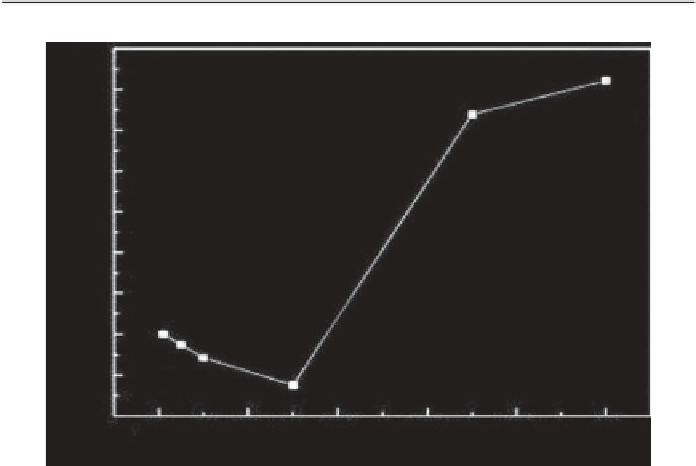
bon pores. It was also found that the addition of salt barely had any effect
on the adsorption capacity of the activated carbon when the solution con-
centration is relatively low. As the solution concentration increased, the
effect of salt addition became more pronounced (see Figure 5.4). At such
high concentrations, the addition of salt was believed to result in a partial
neutralization of the surface positive charge on the activated carbon by
Cl
-
ions and consequent compression of the electric double layer, enhancing
the adsorption capacity of the activated carbon. Also, they hypothesized
Table 5.1
Summary of porous structure of the rice husk-based activated carbons
prepared at various conditions [49,50].
Activation condition
BET SA
(
m
2
g
-1
)
Pore vol.
(
cc.g
-1
)
Micropore SA
(
m
2
g
-1
)
Av. Pore Size
(Å)
750 C, 30 min
1886
0.98
721
20.00
1987
1.32
785
23.47
750 C, 60 min
2721
1.88
1044
25.79
750 C, 90 min
650 C, 120 min
1392
0.70
955
20.20
700 C, 60 min
1759
0.79
1735
17.89
1930
0.97
1090
20.02
750 C, 60 min
1.2
1.1
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.0
0.2
0.4
0.6
0.8
1.0
KCI Concentration (M)
Figure 5.4
Adsorption of RB on rice husk-based activated carbon as a function of
KCl [50]. (Conditions: contact time 2 h; adsorbent dosage 0.8 g/L; [RB] 1.2 mmol/L;
temperature 25°C)


































































Search WWH ::

Custom Search