Environmental Engineering Reference
In-Depth Information
that the
Cl
-
present in the solution would pair with the dye molecules and
reduce the repulsion forces between the adjacent adsorbed dye molecules.
The adsorption behavior of the activated carbon for Rhodamine B dye
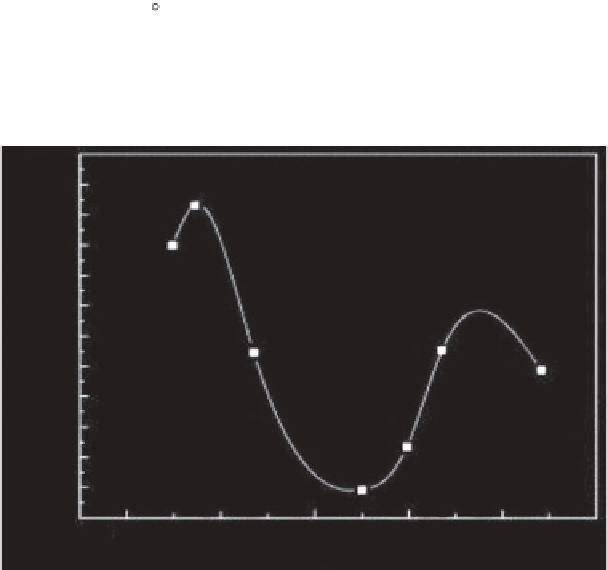
removal at different pH values was interesting. According to Figure 5.5, as
the pH was increased to pH value of 7, the adsorption capacity decreased
and further increase in pH level enhanced the removal capability of the
activated carbon. They attributed the dye uptake reduction at moderate pH
values to the aggregation of monomeric dye molecules and formation of
larger molecules, dimers, which can hinder the diffusion of dye molecules
into the pores. Further increase in the pH level will increase the
OH
-
ions,
which generates a competition between the functional moieties of the dye
molecule and decrease the aggregation.
The physical activation of rice husk using steam has been carried out
by Malik [51]. The rice husk was first carbonized at 400 C for an hour
under air atmosphere and then it was activated at 600 C for 1 h by means
of steam. The surface area obtained was 272 m
2
/g. The adsorption capacity
of the steam-activated carbon for the removal of Acid Yellow 36 was deter-
mined to be around 30
mg.g
-1
. Hameed and El-Khaiary [52] carbonized
the rice straw at 700 C for 2 h under nitrogen atmosphere. The obtained
material had an uptake capacity of around 150
mg.g
-1
for malachite green.
Table 5.2 presents a comparison of the adsorption capacities of the rice
husk-based materials together with the modification procedures.
1.00
0.95
0.90
0.85
0.80
0.75
0.70
0.65
0.60
0.55
0.50
0.45
0.40
2
4
6
8
10
12
pH
Figure 5.5
Adsorption of RB on rice husk-based adsorbent as a function of pH [50].
(Conditions: contact time 2 h; adsorbent dosage 0.8 g/L; [RB] 1.2 mmol/L;
temperature 25°C)

Search WWH ::

Custom Search