Geology Reference
In-Depth Information
8A
(18)
Hydrogen
1
H
1.0079
MAIN GROUP METALS
TRANSITION METALS
Uranium
92
U
238.0289
Helium
2
He
4.0026
1
Atomic number
Symbol
Atomic mass number
1A
(1)
2A
(2)
3A
(13)
4A
(14)
5A
(15)
6A
(16)
7A
(17)
METALLOIDS
Lithium
3
Li
6.941
Beryllium
4
Be
9.0122
Boron
5
B
10.811
Carbon
6
C
12.011
Nitrogen
7
N
14.0067
Oxygen
8
O
15.9994
Fluorine
9
F
18.9984
Neon
10
Ne
20.1797
NONMETALS
2
Sodium
11
Na
22.9898
Magnesium
12
Mg
24.3050
Aluminum
13
Al
26.9815
Silicon
14
Si
28.0855
Phosphorus
15
P
30.9738
Sulfur
16
S
32.066
Chlorine
17
Cl
35.4527
Argon
18
Ar
39.948
3
8B
3B
(3)
4B
(4)
5B
(5)
6B
(6)
7B
(7)
1B
(11)
2B
(12)
(8)
(9)
(10)
Potassium
19
K
39.0983
Calcium
20
Ca
40.078
Scandium
21
Sc
44.9559
Titanium
22
Ti
47.867
Vanadium
23
V
50.9415
Chromium
24
Cr
51.9961
Manganese
25
Mn
54.9380
Iron
26
Fe
55.845
Cobalt
27
Co
58.9332
Nickel
28
Ni
58.6934
Copper
29
Cu
63.546
Silver
47
Ag
107.8682
Zinc
30
Zn
65.39
Gallium
31
Ga
69.723
Germanium
32
Ge
72.61
Arsenic
33
As
74.9216
Selenium
34
Se
78.96
Bromine
35
Br
79.904
Krypton
36
Kr
83.80
4
Rubidium
37
Rb
85.4678
Strontium
38
Sr
87.62
Yttrium
39
Y
88.9059
Zirconium
40
Zr
91.224
Niobium
41
Nb
92.9064
Molybdenum
42
Mo
95.94
Technetium
43
Tc
(97.907)
Ruthenium
44
Ru
101.07
Rhodium
45
Rh
102.9055
Palladium
46
Pd
106.42
Cadmium
48
Cd
112.411
Indium
49
In
114.818
Tin
50
Sn
118.710
Antimony
51
Sb
121.760
Tellurium
52
Te
127.60
Iodine
53
I
126.9045
Xenon
54
Xe
131.29
5
Cesium
55
Cs
132.9054
Barium
56
Ba
137.327
Lanthanum
57
La
138.9055
Hafnium
72
Hf
178.49
Tantalum
73
Ta
180.9479
Tungsten
74
W
183.84
Rhenium
75
Re
186.207
Osmium
76
Os
190.2
Iridium
77
Ir
192.22
Platinum
78
Pt
195.08
Gold
79
Au
196.9665
Mercury
80
Hg
200.59
Thallium
81
Tl
204.3833
Lead
82
Pb
207.2
Bismuth
83
Bi
208.9804
Polonium
84
Po
(208.98)
Astatine
85
At
(209.99)
Radon
86
Rn
(222.02)
6
Francium
87
Fr
(223.02)
Radium
88
Ra
(226.0254)
Actinium
89
Ac
(227.0278)
Rutherfordium
104
Rf
(261.11)
Dubnium
105
Db
(262.11)
Seaborgium
106
Sg
(263.12)
Bohrium
107
Bh
(262.12)
Hassium
108
Hs
(265)
Meitnerium
109
Mt
(266)
—
11
—
Discovered
1996
—
11
—
Discovered
2004
—
11
—
Discovered
1999
—
11
—
Discovered
2004
—
11
—
Discovered
1999
Darmstadtium
110
Ds
(271)
Roentgenium
111
Rg
(272)
7
Cerium
58
Ce
140.115
Praseodymium
59
Pr
140.9076
Neodymium
60
Nd
144.24
Promethium
61
Pm
(144.91)
Samarium
62
Sm
150.36
Europium
63
Eu
151.965
Gadolinium
64
Gd
157.25
Terbium
65
Tb
158.9253
Dysprosium
66
Dy
162.50
Holmium
67
Ho
164.9303
Erbium
68
Er
167.26
Thulium
69
Tm
168.9342
Ytterbium
70
Yb
173.04
Lutetium
71
Lu
174.967
Lanthanides
Note: Atomic masses are
1993 IUPAC values
(up to four decimal places).
Numbers in parentheses are
atomic masses or mass numbers
of the most stable isotope of
an element.
Thorium
90
Th
232.0381
Protactinium
91
Pa
231.0388
Uranium
92
U
238.0289
Neptunium
93
Np
(237.0482)
Plutonium
94
Pu
(244.664)
Americium
95
Am
(243.061)
Curium
96
Cm
(247.07)
Berkelium
97
Bk
(247.07)
Californium
98
Cf
(251.08)
Einsteinium
99
Es
(252.08)
Fermium
100
Fm
(257.10)
Mendelevium
101
Md
(258.10)
Nobelium
102
No
(259.10)
Lawrencium
103
Lr
(262.11)
Actinides
◗
Figure 3.3
The Periodic Table of Elements Only about a dozen elements are common in minerals
and rocks, but many uncommon ones are important sources of natural resources. For example, lead
(Pb) is not found in many minerals, but it is present in the mineral galena, the main ore of lead. Silicon
(Si) and oxygen (O), in contrast, are important elements in most of the minerals in Earth's crust.
spontaneously decay or change to other elements. Carbon 14
is radioactive, whereas both carbon 12 and carbon 13 are
not. Radioactive isotopes are important for determining the
absolute ages of rocks (see Chapter 17).
of only oxygen atoms and is thus an element, whereas the
mineral quartz, consisting of silicon and oxygen atoms, is a
compound. Most minerals are compounds, although gold,
platinum, and several others are important exceptions.
To understand bonding, it is necessary to delve deeper
into the structure of atoms. Recall that negatively charged
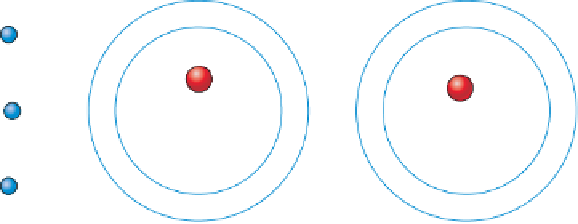
electrons orbit the nuclei of atoms in electron shells. With
the exception of hydrogen, which has only one proton and
one electron, the innermost electron shell of an atom con-
tains only two electrons. The other shells contain various
numbers of electrons, but the outermost shell never has
more than eight (Figure 3.2). The electrons in the outer-
most shell are those that are
usually involved in chemical
bonding.
Two types of chemical
bonds,
ionic
and
covalent
, are
particularly important in min-
erals, and many minerals con-
tain both types of bonds. Two
other types of chemical bonds,
metallic
and
van der Waals
, are
much less common, but are
Interactions among electrons around atoms can result in
two or more atoms joining together, a process known as
bonding
. If atoms of two or more elements bond, the re-
sulting substance is a
compound
. Gaseous oxygen consists
Nucleus
6 p
6 n
6 p
7 n
6 p
8 n
12
C (Carbon 12)
13
C (Carbon 13)
14
C (Carbon 14)
◗
Figure 3.4
Carbon Isotopes Schematic representation of the isotopes of carbon. Carbon has
an atomic number of 6 and an atomic mass number of 12, 13, or 14, depending on the number
of neutrons in its nucleus.
































































Search WWH ::

Custom Search